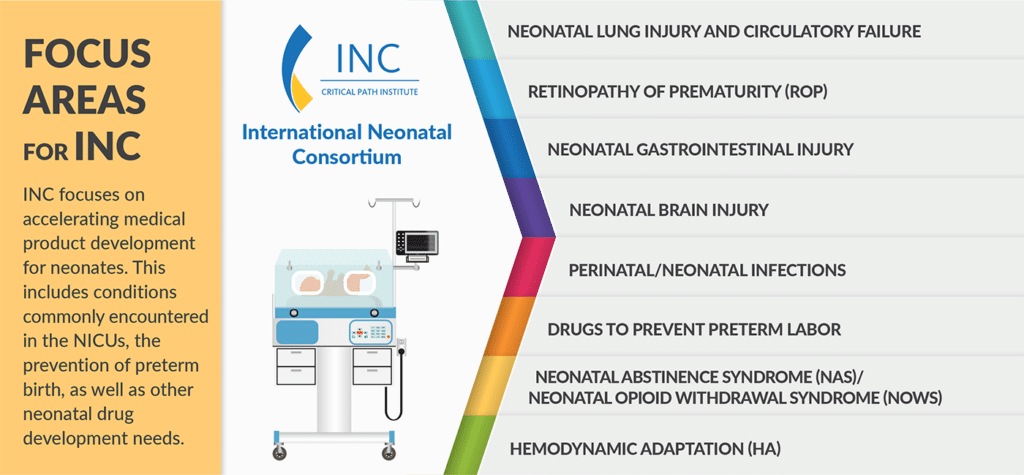
Dr. Janet Woodcock: A Visionary Leader in Regulatory Science and the Inspirational Force behind the Creation of Critical Path Institute
In her nearly four decades with the U.S. Food and Drug Administration, Dr. Janet Woodcock personally transformed the culture of...