Frequently Asked Questions
What is C-Path? How is C-Path funded? How was it formed? You’ve got questions, we’ve got answers. View our FAQs below.
Critical Path Institute, or C-Path, is a global, independent, nonprofit organization dedicated to the generation of actionable solutions to transform the medical product development process. C-Path brings together regulatory agencies, biopharmaceutical firms, universities, other non-profits, and patient groups from around the world to improve public health. Together, these stakeholders work to develop new tools and processes that can accelerate decision-making and medical product development and approval.
C-Path leads collaborations that accelerate drug development, advancing better treatments for people worldwide.
C-Path achieves its mission by acting as an independent, neutral third party to form and lead public-private partnerships of regulatory agencies, biopharmaceutical firms, universities, and patient groups in a pre‐competitive collaboration and sharing of scientific data.
C-Path is a public/private partnership funded by government agencies such as the *FDA, grants from foundations such as the Bill & Melinda Gates Foundation, Michael J. Fox Foundation and the Polycystic Kidney Disease Foundation, as well as fees from industry participants.
*Critical Path Institute is supported by the Food and Drug Administration (FDA) of the Department of Health and Human Services (HHS) and is 54% funded by the FDA/HHS, totaling $19,436,549, and 46% funded by non-government source(s), totaling $16,373,368. The contents are those of the author(s) and do not necessarily represent the official views of, nor an endorsement by, FDA/HHS or the U.S. Government.
In 2004, in response to the FDA’s Critical Path Initiative (CPI) program — a national effort to modernize the process through which FDA‐regulated products are developed, evaluated, and manufactured–an independent 501(c)(3) was founded with the mission to “foster development of new evaluation tools to inform medical product development.” In 2005, a Memorandum of Understanding was created between FDA and Critical Path Institute.
Critical Path Institute (C-Path) is an independent, nonprofit established in 2005 as a public-private partnership, in response to the FDA’s Critical Path Initiative. With dedicated team members located throughout the world, C-Path’s global headquarters is located in Tucson, Arizona and C-Path’s Europe subsidiary is headquartered in Amsterdam, Netherlands. Each performs program work through consortia and separate initiatives. View all initiatives here
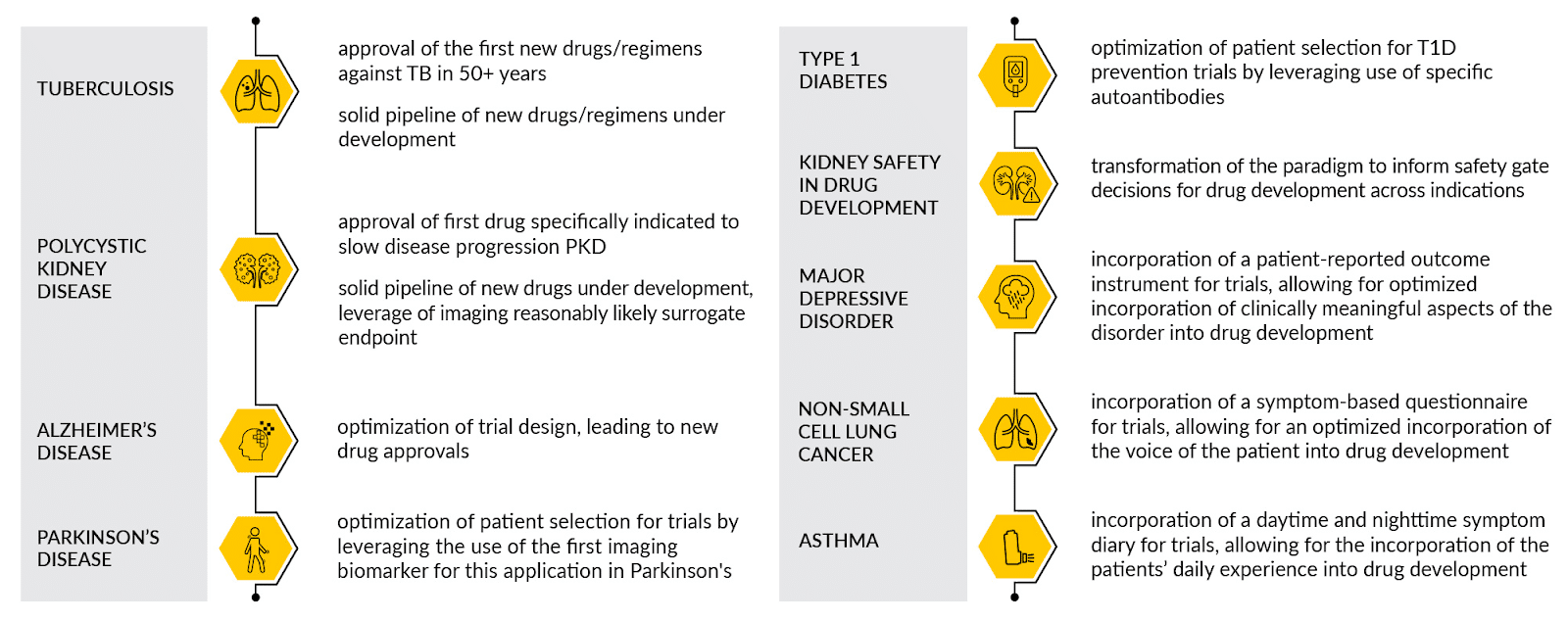
C-Path’s collaborations advance the generation of actionable solutions that help transform the drug development process for the following indications:
| Alzheimer’s disease | Non-Small Cell Lung Cancer |
| Asthma | Organ Transplant |
| Ataxias, including Friedreich’s Ataxia | Parkinson’s Disease |
| Depression | Polycystic Kidney Disease |
| Duchenne Muscular Dystrophy | Rare Diseases |
| Functional Dyspepsia | Rheumatoid Arthritis |
| Huntington’s Disease | Tuberculosis |
| Irritable Bowel Syndrome | Type 1 Diabetes |
| Multiple Sclerosis |
We also have expertise in the following:
| Biomarker qualification | Drug repurposing |
| Clinical outcome assessment qualification | Drug safety and drug testing |
| Clinical trial simulation tools | Evidentiary considerations for biomarker qualification |
| Data collaboration | Patient-reported outcomes and electronic patient-reported outcomes |
| Data standards development | Quantitative medicine – modeling and simulation |
| Database and data repository management | Regulatory science |
Please visit our Core Competencies page for more information.
- Our IRS Form 990 can be found at GuideStar.org.
- To obtain printed copies of our audited financial statements or if you have any questions, please contact us at info@c-path.org.
- Click here to view our list of annual reports.
Yes, we currently have the highest possible ratings on GuideStar.org (Platinum) and charitynavigator.org (100/100).
Our Federal Tax ID number is 20-1991334.

