The FDA has issued a Letter of Support to the Predictive Safety Testing Consortium (PSTC) to encourage the further study and use of a set of translational safety biomarkers to detect and monitor drug-induced pancreatic injury (DIPI). The set of biomarkers are four micro RNAs (miRNAs) under investigation for DIPI are miR-216a, miR-216b, miR-217, and miR-375.
The translational pancreatic safety biomarkers (miR-216a, miR-216b, miR-217, and miR-375) will be used in conjunction with amylase and lipase to aid in the detection of DIPI in Phase 1 clinical trials when there is demonstrated monitorability of induced damage in nonclinical studies and sufficient safety margins or when there is a priori concern that a drug candidate may induce DIPI due to known structural class similarities or identified target liability concerns.
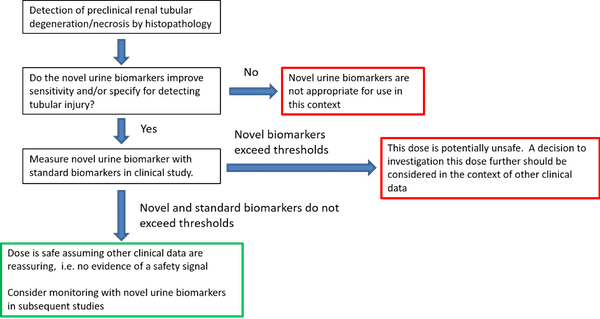
Novel biomarkers of DIPI used in combination with currently available biomarkers could play an important role in guiding safety assessment in phase I clinical trials. Although the standard markers of pancreas injury, amylase and lipase, are currently the clinically accepted biomarkers for the diagnosis of acute pancreatitis (IAP/APA evidence-based guidelines for the management of acute pancreatitis, 2013), it is widely acknowledged that they are not sufficiently sensitive nor specific to guide dosing-related decisions in clinical trials (Lee and Papachristou, 2019; Ismail and Bhayana, 2017).
We support PSTC’s initiative to encourage the voluntary and complementary use of these miRNAs in conjunction with amylase and lipase as exploratory nonclinical and clinical biomarkers of DIPI. We also support PSTC’s generation of additional nonclinical toxicology data and plan for exploratory early clinical studies to enable future formal qualification of these safety biomarkers.
MiRNA molecules are short non-coding sequences ranging from 19 to 25 nucleotides in length, which are involved mainly in the post-transcriptional modulation of gene expression. A diverse array of functions including cellular signaling, cell growth and differentiation and apoptosis have been described for miRNAs. In humans, approximately 2,600 miRNA genes are currently known. Information on miRNA identification and nomenclature can be found at www.mirbase.org. Because of the relative abundance of miRNAs in specific tissues, they are increasingly being recognized as leakage biomarkers indicative of tissue injury (Bailey and Glaab, 2018; Schraml et al., 2017). A number of candidate miRNAs have been identified and studied in association with pancreatic acinar injury in nonclinical species (Erdos, 2020; Usborne, 2014), including miR-217, miR-216a, miR-216b, and miR-375.
Member companies of the PSTC PIWG have conducted studies and assessed pancreas-specific miRNAs to determine their performance in monitoring drug-induced toxicities. Toxicants targeting Exocrine/Acinar Predominant Injury (caerulein, caerulein perfusion, cholecystokinin, L-arginine, cyanohydroxybutene, DL-Ethionine, sodium taurocholate, Dibutyltin dichloride, Ethanol + Cyclosporin A + caerulein, ductal retrograde trinitrobenzene sulfonic acid, and proprietary drug candidates) and Endocrine/Islet Predominant Injury (streptozotocin and proprietary drug candidates) assessed the performance of the miRNAs to detect DIPI in rats (all toxicants) and dog (only cholecystokinin). The studies supporting this Letter of Support were published in peerreviewed scientific journals. These pancreas-specific miRNAs have been evaluated as biomarkers of DIPI and show promise to add value to the interpretation of amylase and lipase in monitoring acute pancreatic injury defined as acinar cell degeneration/necrosis.
More experience with the use of this biomarker in clinical studies would be useful to determine its utility more accurately for detecting and monitoring drug-induced pancreatic injury. MicroRNA thresholds have yet to be determined in clinical samples. Assessment will be in conjunction with amylase and lipase and not be used independently for decision making. Future studies will determine the clinical thresholds and the interpretation of results including performance metrics. We encourage exploration of these miRNAs (miR-217, miR-216a, miR-216b, and miR-375) used, in conjunction with amylase and lipase, to aid in the detection of DIPI in Phase 1 clinical trials when there is demonstrated monitorability of induced damage in nonclinical studies and sufficient safety margins or when there is a prior concern that a drug candidate may induce DIPI due to known structural class similarities or identified target liability concerns.
Any groups (academia, industry, government) that would like to join in this effort or have information or data that may be useful can contact Mr. Nicholas King (nking@c-path.org) or view the PSTC website.