C-Path’s CURE Drug Repurposing Collaboratory Aims to Document 1,000 Cases on the CURE ID App for Long COVID Awareness Day
TUCSON, Ariz., March 15, 2024 — Critical Path Institute’s (C-Path) CURE Drug Repurposing Collaboratory (CDRC), a leading advoc


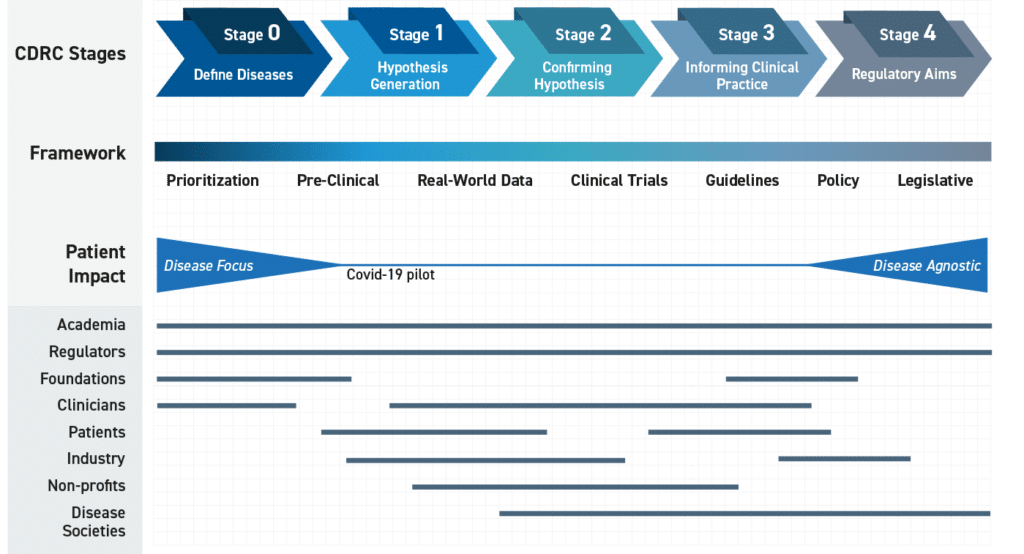
CDRC is designed to capture real-world clinical outcome data to advance drug repurposing and inform future clinical trials for diseases of high unmet medical need.
There are approximately 10,000 known diseases, but only 2,500 of them are addressed by FDA-approved drugs. That leaves around 7,500 diseases without FDA approved treatment, many of which are rare diseases, diseases in pregnant women, children and neonates, as well as neglected tropical diseases and emerging/reemerging infectious diseases. Many existing drugs might work to treat these diseases, but often there are no commercial or regulatory incentives – or there are actual disincentives — to do the extensive clinical testing required to show they are effective.
In response to these challenges, the CURE Drug Repurposing Collaboratory (CDRC) was founded to explore whether already marketed drugs can be repurposed for diseases that are not commercially attractive. CDRC is strongly interested in capturing data from diverse populations, including pediatric patients and pregnant women, as well as under-served populations.
As part of this process, a roadmap will be developed for the efficient generation of evidence, in order for FDA to update labeling. This includes creating a path of potential scenarios, depending upon the context of the specific indication, unmet patient needs, the engagement of the drug sponsor or the first NDA holder and the status of current legislation. The data gathered through the CURE ID app can be used to inform the design of clinical trials, to evaluate the effectiveness and safety of a marketed therapy intended for repurposing, or to support treatment guideline development.
The path chosen will depend on the drug(s) in question (patented vs generic), the disease epidemiology and natural history (availability and procurement of sufficient data), and the existing legislative mechanisms (sponsor, payer, and/or patient support). Although many of the challenges of drug repurposing are global, regulators need to address these locally and within the existing regulatory systems. As regulators globally pilot programs, an ability to provide lessons learned and potentially share information to harmonize processes will enable pharma and manufacturers to better align by reducing complexity, lowering costs, and ensuring safe and effective treatments for all patients.
The Collaboratory, led by Critical Path Institute (C-Path), works in a transparent, open forum, with a diverse set of global stakeholders including, but not limited to, clinicians, scientists, U.S. Health and Human Services (HHS) agencies, non-government organizations, foundations and societies in order to:
View the detailed agenda here.
View the speaker bios and headshots here.
View the Poster Presentations here.
View the presentations from Day One here
View the presentations from Day Two here
View the presentations from Day Three here.
View the recording from Day Two here.
View the recording from Day Three here.
Day 1 Panel Discussions: Session 1: Rare Diseases
Moderators: Matt Might (UAB) and Shira Strongin (FDA)
Panelists: Andy Crouse (UAB), Ethan Perlstein (Perlara), Eva Morava-Kozicz (Mayo), Lisa Schill (RASopathies Network), Omid Karkouti (Rarebase), Clare Thibodeaux (Cures Within Reach), Sandra Sermone (ADNP Kids Research Foundation)
Session 2: Rare Oncology
Moderators: Leslie Doros (FDA) and Sonia Singh (FDA)
Panelists: Bill Tap (MSKCC), Denise Reinke (U Michigan), Kris Ann Schultz (Children’s MN), Pan Pantziarka (Anticancer Fund), Breelyn Wilky (U Colorado)
Day 2 Panel Discussions: Session 3: Special Populations
Moderators: Matt Laughon (UNC) and Kate Borkowski (FDA)
Panelists: Anup Challa (Vanderbilt), Rachel Greenberg (Duke), Mili Duggal (FDA), Matt Robinson (Hopkins), Khyzer Aziz (Hopkins), Perdita Taylor-Zapata (NICHD), Kanwaljit Singh (INC), Prabha Viswanathan (FDA), Genny Taylor (UNC), David Kimberlin (UAB), Jason Lang (Duke), Barbara Goodman (Cures Within Reach)
Session 4: Regulatory
Moderators: David Simon (Harvard) and Marco Schito (C-Path)
Panelists: Jonathan Darrow (Harvard), John Liddicoat (Cambridge), Sundeep Agrawal (FDA), Daniel O’Connor (MHRA), Lydie Meheus (Anticancer Fund), Heather Stone (FDA), Perdita Taylor-Zapata (NICHD), Nitin Bagul (TGA), César Hernandez Garcia (AEMPS Spain), Momir Radulović (Slovenian Medicines Agency), Agnes Klein (Health Canada)
Day 3 Panel Discussions: Session 5: Electronic Health Records
Panel 1 Moderators: Smith Heavner (C-Path) and Aysun Tekin (Mayo Clinic)
Panelists: Rahul Kashyap (VIRUS Registry), Matt Robinson (JHU), Paul Nagy (JHU), Matt Roe (Verana Health), Laura Merson (Oxford)
Panel 2 Moderator: Laura Merson (Oxford) and Smith Heavner (C-Path)
Panelists: Will Stevens (RECOVERY Trial, University of Oxford), Ann-Marie Mallon (NHS Digital), Kalynn Kennon (IDDO), Miguel Pedrera Jimenez (Hospital 12 de Octubre, Madrid, Spain)
CDRC 2021 Annual Meeting: The Collaboratory
Session 1
Session 2
Session 3
Session 4
Session 5
C-Path’s Cure Drug Repurposing Collaboratory will host a three-day annual meeting in a virtual format, November 16-18, 2021.
Together, with key stakeholder groups including clinicians, researchers, foundations, nonprofits, patient advocates and regulators, CDRC will host a public webinar this November to discuss how the Collaboratory can capture real-world data that generate hypothesis regarding treatment efficacy using repurposed drugs to address diseases of high unmet clinical need. The meeting will include key opinion leaders from numerous disease communities as well as experts on automated EHR data extraction and ways in which these resources can be leveraged to develop partnerships to inform large adaptive platform trials.
What to expect:
Day One will be hosted by the Rare Diseases and Rare Oncology Coordinating Committees.
Topics will include:
Day Two will be hosted by the Regulatory, Legislative and Policy working group and the Special Populations Coordinating Committee. Topics will include:
Day Three will include sessions dedicated to electronic health record data extraction for COVID-19. Topics will include:
The Role of Drug Repurposing in Pregnancy
USA Today | Treatment for COVID-19 is better than a year ago, but it still has a long way to go
Cures Within Reach | Cures Within Reach Names Two Recipients of its 2021 Patient Impact Awards
CNN | The FDA’s new app for frontline doctors could help discover Covid-19 treatments faster
Regulatory Focus | UPDATED: FDA’s CURE ID app gets COVID-19 refresh
Medscape | Clinicians Urged to Use CURE ID to Report COVID-19 Cases
IDSE Special Edition | CURE ID Site Gains Traffic From Experimental Coronavirus Rx Reports
For internship opportunities please email CDRC@c-path.org
Literature Review and Data Intern
Communications and Outreach Intern
Download the CURE ID app at (https://cure.ncats.io/) and begin submitting cases today. It takes a couple of minutes and every case report counts.
For questions or additional information about participating in CURE ID and the CDRC, please email cdrc@c-path.org.
A significant number of accomplishments were completed in Year 9 across several diseases (infectious, non-communicable), clinical trials (decentralized outpatient, inpatient embedded in clinical practice), and the development of tools to automate the extraction of quality key data variables from electronic health records. Specifically, this includes:
* The CURE ID mobile app is an FDA-NCATS collaboration to build an internet-based repository that allows the global clinical community to report novel uses of existing drugs to treat diseases through a website, a smartphone, or other mobile device. The repository captures the clinical outcomes when drugs are used for new indications, in new populations, in new doses, or in new combinations.
The CURE Coordinating Committees will begin by initiating a comprehensive landscape analysis for diseases where drug repurposing is being utilized for patient treatment of diseases with high unmet medical need. The Collaboratory will finalize a list to identify disease and treatment combinations where FDA approved treatments do not exist or are inadequate. This may include categories encompassing additional infectious diseases (e.g., emerging diseases, drug resistant infections and neglected diseases), rare diseases (e.g., non-infectious, oncology) and diseases in special populations (pregnant women, neonates, pediatrics). Once completed, a set of pre-defined criteria will be finalized that will allow the list to be prioritized. Examples of criteria may be the availability of drug treatment data or the identification of an active and collaborative disease research and advocacy community. In addition, the group will be charged with defining the key questions that the analytics team will be requested to address, including any thresholds identified based on quantitative analysis of values captured. This will allow the application of predefined criteria to identify analysis output that could be flagged for the CURE Drug Repurposing Collaboratory to review. Finally, the CURE Drug Repurposing Collaboratory stakeholders will draft standard operating procedures to define parameters necessary to select a drug to go through a defined repurposing effort based on existing data, level of evidence (e.g., existing guidelines), as well as the legislative and regulatory climate.

There are many diseases and conditions where regulatory approved therapies are either absent or unsatisfactory and prescribers are forced to repurpose existing drugs for patients with difficult-to-treat diseases. This produces two significant challenges facing all communities relying on re-purposed drugs:
Initially, the failure by the pharmaceutical industry to invest in and pursue potential new uses is an innovation problem: there may be perceived barriers and firms may lack the necessary incentives to research and develop new uses of existing drugs approved for other indications, especially after they become generic. In addition, efforts have been initiated to capture a small number of deidentified data elements from data that is currently being captured (see data tools below) to ultimately populate the publicly accessible CURE ID data platform to generate hypothesis regarding the efficacy of repurposed drugs.
The problem of off-label use is one of both safety and efficacy: off-label uses are not supported by the same level of evidence as approved uses and are used at greater risk, both physically and monetarily, to the patient. Under these circumstances, physicians work with limited information to treat patients to the best of their abilities. However, as they do, the systematic collection of this anecdotal information could be utilized to inform others in similar situations.
Data capture tools:
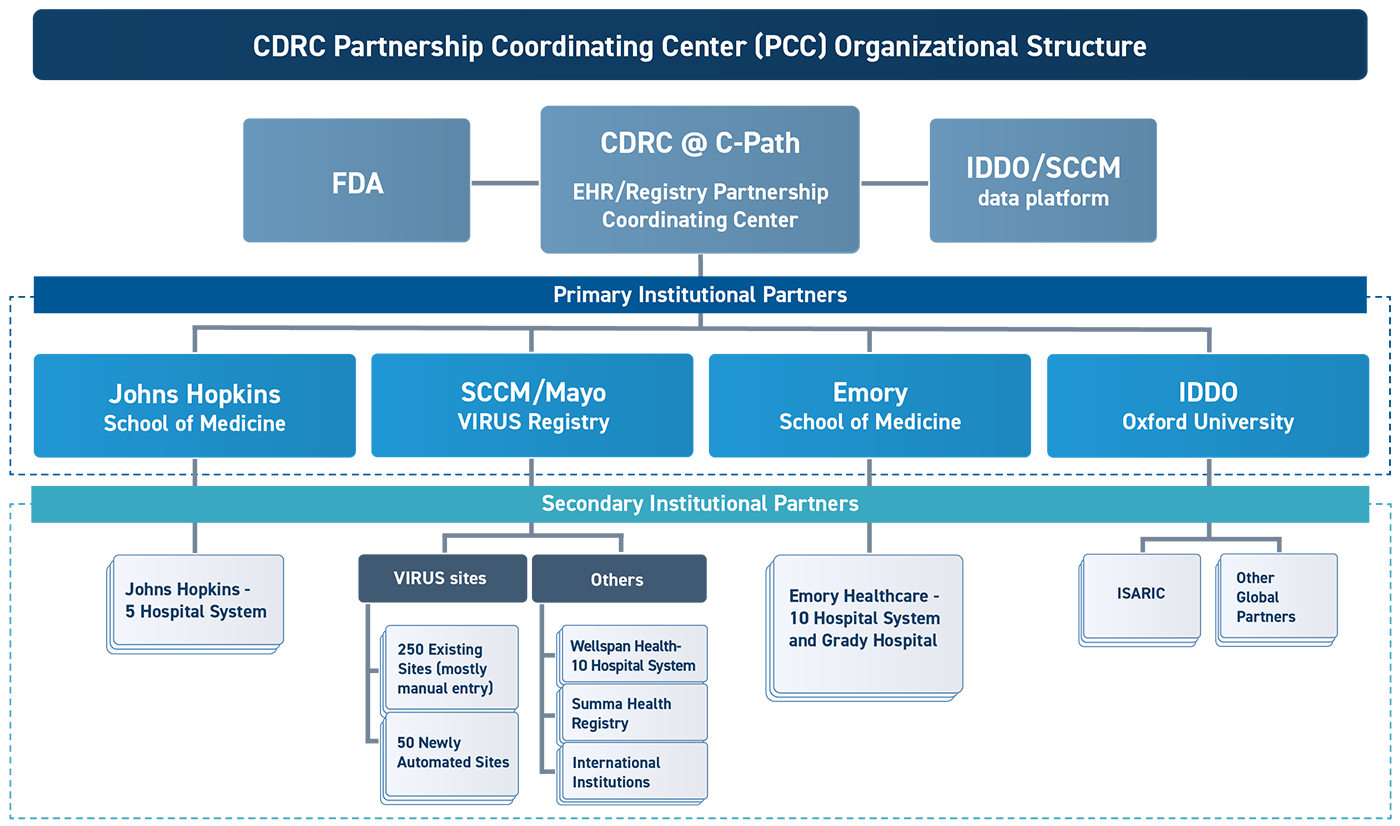
CURE ID has secured a $8.2 million grant through the HHS Assistant Secretary for Planning and Evaluation (ASPE) Patient-Centered Outcomes Research Trust Fund (PCORTF) to automate the extraction of a limited subset of deidentified data from sites around the world for the purpose of the following:
This infrastructure will be built for COVID-19 but will be designed in a sustainable manner so that it can be promptly deployed for future outbreaks of existing and emerging infectious diseases as well as leverage the infrastructure for diseases in the interpandemic period. This will provide real-time access to the global clinical experience of repurposing drugs when there is an immediate need to identify potential existing treatments in the absence of novel drug development.
The expanded CURE ID platform will include hundreds of thousands of cases, making it substantially larger than at present, given that cases are entered manually. This large collection of cases will enable the clinical, research and regulatory communities to identify signals of potentially safe and effective COVID-19 treatments from amongst the armamentarium of existing FDA approved therapeutics. These signals will enable the generation of robust clinical hypotheses and by doing so, may accelerate the identification and development of effective treatments.
While it has been clear for decades that the systems for clinical research in the US are broken, the COVID-19 pandemic has laid bare the limitations of these systems for answering the most important research questions to guide clinical practice in a timely, coordinated and efficient manner. A different approach is needed that seeks to integrate clinical research into clinical practice and make it easy to prioritize and rapidly answer the most pressing clinical questions.
CDRC’s vision for such an approach includes the development of a standing infrastructure for conducting research embedded within clinical practice in three different care settings. It proposes to take advantage of the scientific rigor afforded by randomization of trial participants to different treatment arms, while making the conduct of the trial so much less burdensome to healthcare providers, that it is possible for them to participate with little added effort.
The goal would be to conduct a series of trials under a common randomized clinical trial platform structure using repurposed and novel medications to treat COVID-19 and other serious illnesses.
The initiative will encompass a series of three separate but linked platform trial infrastructures:
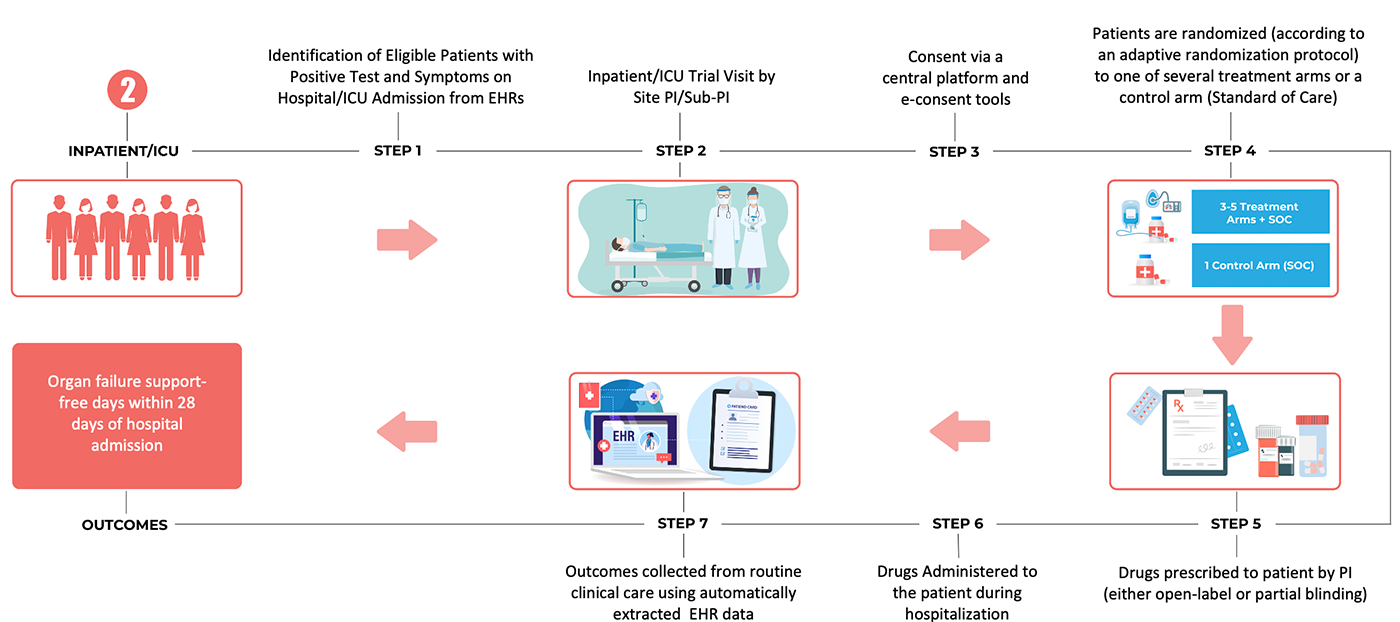
2. An acute inpatient trial infrastructure, based on embedding the trial within clinical practice through use of electronic health record (EHR) systems
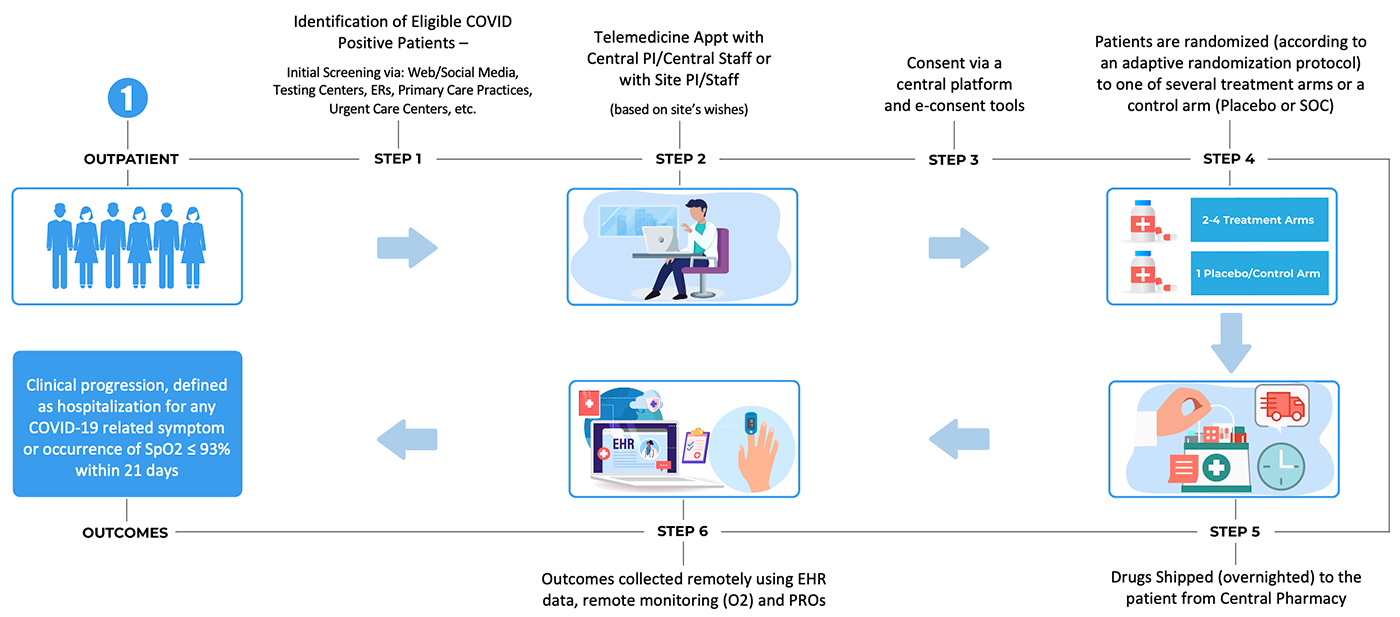
3. A chronic outpatient trial infrastructure for long-term follow-up of chronic conditions (e.g., post-acute sequelae of COVID-19, post-ICU syndrome, etc.), leveraging telemedicine, drugs delivered by mail and an emphasis on electronic patient-reported outcomes
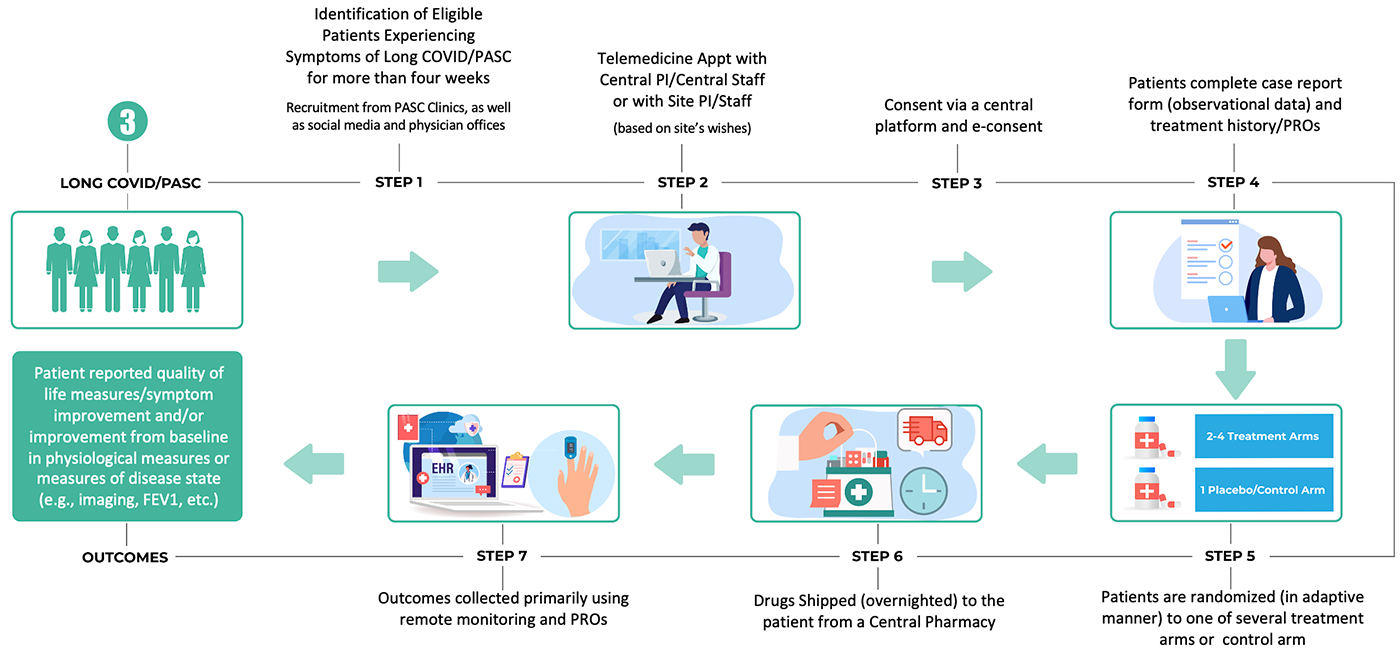
The goal is to design these trial platforms to be flexible and adaptable enough to be used both in the context of future outbreaks, as well as for study of diseases and health conditions in non-emergency settings.
The trials would all use master protocols with adaptive designs to enable the efficient and sustainable, but scientifically rigorous, evaluation of treatments, with particular emphasis on diseases with high unmet medical needs (that are not well-served by commercial interests) and the use of drugs that are already approved by the FDA or another stringent regulatory authority. They would utilize adaptive randomization so that treatments could be stopped due to futility and other drugs could be added to enable study of additional treatment options.
With nearly a third of all known diseases having approved drugs, a significant proportion of diseases have limited or no approved treatment options. One reason for the lack of treatment is that many of these diseases are not determined to be financially feasible for for-profit pharmaceutical companies to pursue. Diseases which still lack a medical treatment to slow, stop, or reverse their course result in mortality, but also result in distress, morbidity and reduced quality of life. As a result, doctors often prescribe drugs to patients off-label using their professional judgment or follow treatment guidelines which may lack sufficient safety or efficacy data. The current regulatory relabeling system works well for drugs on patent but the number of drugs that companies pursue supplements to existing labels sharply declines several years before losing patent exclusivity. Once a drug has become a generic, there are no incentives for manufacturers to pursue label supplements and the current system prevents others to pursue label updates even if they have relevant clinical data. In addition to financial incentives there are a host of other challenges that must be debated and discussed including reimbursement, patent law, existing legislative tools and new regulatory pathways.
To address these issues, a stakeholder group has been convened under the auspices of CDRC to gather stakeholder input to describe and identify the barriers to supplemental drug labels in the case of repurposed drugs. The real-world data gathered through the CURE ID app can be used to inform the design of clinical trials, evaluate the effectiveness and safety of a marketed therapy intended for repurposing, or support treatment guideline development. In partnership with regulators and in collaboration with other stakeholders, a strategy will be defined to promote a regulatory pathway for repurposed drugs through supplemental drug labeling that benefits patients and public health needs.
For questions or additional information about participating in CURE ID and the CDRC, please email CDRC@c-path.org.
CURE ID is expanding! We’re making it possible for patients and their care partners to share their thoughts on treatments. Right now, doctors share information, but we want to hear from you too. Your stories can help us understand how well medicines work, even the things we can’t see in tests or pictures.
Videos
Sarcoma Collaborators

Download the CURE ID app at (https://cure.ncats.io/) and begin submitting cases today. It takes a couple of minutes and every case report counts.
For questions or additional information about participating in CURE ID and the CDRC, please email cdrc@c-path.org.
More information coming soon!

Obtaining and Using TPOXX Tecovirimat for Treatment of Monkeypox
An overview of how to obtain and use Tecovirimat (TPOXX) during the 2022 monkeypox public health emergency under Expanded Access Investigation New Drug (EA-IND). Dr. Raghav Tirupathi, an infectious diseases expert and Clinical Director for Infectious Diseases at CDRC, shares his experience and insight.

The Cure Drug Repurposing Collaboratory (CDRC) will provide a forum for the exchange of clinical practice data to inform potential new uses of existing drugs for areas of high unmet medical need, advancing research in these areas. The Collaboratory will also create a network connecting major treatment centers, academic institutions and researchers, private practitioners, government facilities and health care professionals around the world.
CDRC is a public-private partnership initiated in June 2020 by C-Path and the U.S. Food and Drug Administration (FDA) in partnership with the National Center for Advancing Translational Sciences (NCATS), part of the National Institutes of Health (NIH). The collaboration CDRC is connected and working with, the FDA-NCATS CURE ID* platform.
The initiative includes emerging/reemerging diseases, including COVID-19, anti-microbial drug resistant infections, neglected infectious diseases as well as rare oncology diseases where there are limited treatment options. The Collaboratory is strongly interested in capturing data from diverse populations including pediatric and pregnant women.
CURE ID is an internet-based repository that lets the clinical community report novel uses of existing drugs for difficult-to-treat infectious diseases through a website, a smartphone or other mobile device. The platform enables the crowdsourcing of medical information from health care providers to facilitate the development of new treatments for neglected diseases.
CURE ID is a collaboration between the FDA and the National Center for Advancing Translational Sciences (NCATS), part of the National Institutes of Health (NIH). FDA and NIH are also collaborating with the World Health Organization and the Infectious Disease Society of America to assess the global utility of the CURE ID.
No, this is data collected as part of the treatment that is done at the physician’s discretion during routine clinical care. The data based on this clinical care is simply captured after the fact and shared in a completely de-identified case report form.
The methods are meant to be seen as complimentary and part of the broader real-world evidence (RWE) initiatives at FDA and NIH. Some of the key differences that set this apart from N3C, the Evidence Accelerator, and others is that: 1) the CURE ID platform is global and would seek data from outside the US, in addition to national data sources; 2) data will be combined from several different types of sources – EHRs, but also registries, clinician-submitted cases, and cases extracted from the published literature; 3) the data would be completely accessible at the de-identified patient level to any health care professional or researcher, without a data use agreement, making it much more accessible; and 4) the information collected is quite high-level and uncomplicated in the form of a completely de-identified case report form. Similarities and differences between N3C and CURE ID:
| Issue | N3C | CURE ID |
| Access by non-institutional members | Restricted access: only allows access to synthetic data for non-institutionally affiliated users | Open access: allows access to complete (real) de-identified data set for all users |
| Data use agreements | Required to access data | Not required to access data |
| Purpose of data collection | COVID-specific to answer a wide range of potential research questions requiring complex and detailed information with which to conduct analyses | Not disease specific to answer limited range of high level and priority treatment questions; limited detail required; purpose is for “rough cut” hypothesis generation |
| Scope of content | EHRs only | Combine data from EHRs, smaller registries, clinician-submitted case reports, and data extracted from the published literature |
| Geography of data collection | US (domestic) only | Global |
| Training | Requires 60-90 minutes of training | Does not require any training |
| Data exchange | Shares actual EHR data using one of several common data models | Data is translated from the EHRs to the de-identified CURE ID case report form at the institutional site, facilitating ease of information sharing |
| Data location | All access and analysis takes place within the N3C Data Enclave | Data access is available on the CURE ID website/app, but the entire dataset can also be downloaded to facilitate research |
By entering case studies into the app, health care providers have the opportunity to help uncover new uses for approved drugs and help people in need get life-saving treatments. Situations with disappointing results are equally important to enter, so that others can learn from those experiences and not replicate that treatment protocol. Health care providers may have data already. They are invited to join the CDRC disease working groups to help identify specific questions that they would like answered. Collected data metrics can be made available to this community and updated over time. People who contribute to the CURE ID app may also get recognition in the form of a certificate from FDA and NIH.
Even if health care providers don’t have an experience to share, they can also use the app to search for novel uses of drugs and gain valuable insight, which can inform their future practice and clinical endeavors. People can also use the Discussion Forum to connect with others and share similar experiences and questions.
This is an ongoing effort initiated in June 2020 and will be a multi-year effort. Please contact CDRC, CDRC@c-path.org, for further information.
CURE ID continues to expand the number of clinicians involved, specific diseases it focuses on and methods of data capture (such as automating collection from EHRs and registries). The long-term goal is for the public-private partnership (CDRC) to oversee and coordinate collaboration between stakeholders.The mission of the group is to generate real-world evidence regarding the safety and effectiveness of the use of off-label drugs with high public health impact and low commercial viability/interest for drug repurposing.
Visit the CURE ID website here. You can download the App from the App or Play Store by searching for “CURE ID.” A videocast of the Repurposing Generics workshop at which CURE ID was launched is available here. More information on the CURE ID platform is available on the FDA website here. To join the CURE Drug Repurposing Collaboratory, please send inquiries to CDRC@c-path.org.
View a demonstration and walk-through of the app and the entry of a case report here — Data extraction example.
To enter a case the user must a minimum of seven required questions. Once they do so they have the option to either “Quick Submit” or they can continue and answer any of the remaining 20 optional questions in the complete submission. They also have the option to save their case without submitting and return to it at a later date, or to submit it and update it later by going in and editing the case. If they wish, they are provided an option to anonymize a particular submission so that their name will not be visibly associated with the case or discussion.To enter a discussion the user can either specify a particular disease or choose “disease not specified.” They will then enter a free text title and body of the discussion post, which has a limit of 4,096 characters.
In addition, users can comment on case reports or discussion posts submitted by other users.
Case reports and discussions are uploaded in real-time and reviewed for quality and appropriateness within 12-24 hours by CURE ID moderators. Moderators may make small adjustments (i.e., remove personally identifiable information (hat is accidentally included) or seek additional information or clarifications from the report’s author. They will comment on the post with any changes that are made. If a report is deemed to be inappropriate or otherwise requiring deletion, it will be reviewed by a second moderator. If the second moderator concurs, the post may be removed, and the report’s author will be emailed with an explanation of the decision.
If a user enters a case report the data is all entered in a completely de-identified fashion so as not to trigger any patient privacy concerns. Users should be certain that they don’t include any public health information (PHI) in the free-text entry fields. Because the data was collected for the purpose of clinical care and is shared in a deidentified manner, it is not considered research and thus, patient consent is not required.
Interested users who are licensed health care providers complete a very brief registration where they set up an email and password and state their medical qualifications. Once they have registered, a user can now explore the case reports, discussion posts, and clinical trials in the platform; they can submit their own case report or discussion post; or they can view the Newsfeed.Those who can currently enter data are clinicians, physicians, nurses, nurse practitioners, etc.
If you are not a health professional but can contribute to discussions (i.e., as a veterinarian, epidemiologist, infectious disease researcher, etc.) please contact CURE ID or CDRC to find out how you can download and use the app.
The platform is only open to licensed health care professionals so that clinicians will feel comfortable sharing their treatment experiences – both positive and negative – in a safe, clinical community. Registered users have access to all of the data and can contact the help desk for a download of all of the data in the database if they wish to conduct their own research with it.
The data in CURE ID will be digested by CDRC through a dedicated bioinformatic pipeline and the insights generated will be broadly shared with the public via CURE ID once the results have been reviewed and vetted within the Collaboratory.
Shared publicly means that once your data is transformed into the CURE ID format (and it is certain that any PII has been removed), the case report with patient-level data is openly displayed to users who have signed up to use the platform. The information is in addition to the other sources of case reports (electronic health records, registries, literature, etc.).
Yes, the deidentified data as translated into the case report form would be displayed in CURE ID and thus shared with other app users. One can also request to download the data. The intent here is to share what may be working but also what may not be working with other physicians who are desperately looking for ways in which to treat patients in the absence of data.
Yes, we are collecting AEs and have set up a system to automatically share and upload any case reports that contain AEs with the FDA Medwatch AE reporting platform.
No, not at all. The data won’t be combined with data on the same patients or link them, the data will be combined with other sources of case reports, such as registries and clinician-submitted cases to calculate an aggregate of patients treated with the same drug or drug regimens.
Users do not have to sign a data use agreement of any kind. (see case report form for the snapshot of data elements being collected) during the course of routine clinical care (and therefore not human subjects research), ensuring that the data transferred is HIPAA compliant and completely de-identified per Safe Harbor requirements, the data can be transferred without a data use agreement. Typically, the individual or institution would translate the patient’s record or EHR/registry data into the CURE ID case report form at their site, before sending the de-identified case report form to the CURE ID platform. The data is then made openly accessible to all qualified clinicians and researchers who register on the site.
At the present time, we are asking contributors to share data broadly which negates the rationale to implement a data use agreement since their data would be shared publicly through the CURE ID platform without restrictions.
Yes, it’s clinical data, but high level – for example: was the patient diagnosed with COVID-19 by PCR? After said diagnosis, what drugs were given to the patient? What was the outcome of treatment (did the patient die, admitted to ICU, discharged from ICU to floor, discharged from hospital, etc. – translated to the options of “patient improved,” “patient deteriorated,” etc.). The CDRC and CURE ID team can assist in creating a translation infrastructure which would then automate the transfer of information from your records to the CURE ID Case Report Form, and then we would upload the case reports and they would be reviewed by clinicians on our staff for quality and accuracy.
Historically, yes, we have collected standardized CURE ID case report forms directly from clinicians and other health care providers using the electronic platform we developed. We have also used this same case report form as an extraction tool to review and enter published case reports into the database. View this as a transformation of your data to populate the simplified CURE ID case report form at your site. An automated system could be used to transform the EHR data at your institutional site into the CURE ID case report form, which would then be sent to NCATS for inclusion on the server.
The ideal scenario would be to use automation to extract the information from the EHRs into the CURE ID template, using a series of rules and translations. However, we realize that that may not be possible. If that is the case, we would be happy to explore using data entry specialists, hired by your . It is also possible, that CURE ID could use a combination of the two – where some data is able to be automatically extracted from the EHRs into CURE ID, and other information requires a human.The development of AI tools will help to translate the data into the case report form, but have human curators review a prespecified portion to ensure that reports are being transformed correctly and that it is of high quality. Some tools have already been developed but will need to be refined to address this specific issue. Collaborators from USG resources and the informatics research community can be accessed to help address the challenges.
No, health care providers are able to enter cases that utilize generic and/or branded drug products. It should be noted, however, that submitting a case report to CURE ID is not a substitute for reporting required under public health or legal requirements. There has been an emphasis on off-patent drugs because they are cheaper to purchase and, in theory, could be easier to access. If a drug, disease, or organism you are interested in reporting a case on is not in the drop-down menu of the app, it can be added by typing the text and then hitting enter.
Off-patent drugs are regulatory-approved medical products that no longer have patent protection. Repurposing approved drugs for new clinical indications can potentially offer an efficient drug-development pathway for treatments of diseases and conditions that have few or no therapeutic options. Advantages of using off-patent drugs in this manner includes cost savings, in that such drugs are often available from a variety of manufacturers. In this same context, however, smaller profit margins may exist for companies that repurpose drugs in the same dosage and formulation for new indications, providing less incentive for repurposing.
No, the focus is on the treatment of this particular infection.
To enter a case the user must a minimum of seven required questions. Once they do so they have the option to either “Quick Submit” or they can continue and answer any of the remaining 20 optional questions in the complete submission. They also have the option to save their case without submitting and return to it at a later date, or to submit it and update it later by going in and editing the case. If they wish, they are provided an option to anonymize a particular submission so that their name will not be visibly associated with the case or discussion. To enter a discussion the user can either specify a particular disease or choose “disease not specified.” They will then enter a free text title and body of the discussion post, which has a limit of 4,096 characters.
In addition, users can comment on case reports or discussion posts submitted by other users.
Case reports and discussions are uploaded in real-time and reviewed for quality and appropriateness within 12-24 hours by CURE ID moderators. Moderators may make small adjustments (i.e., remove personally identifiable information that is accidentally included) or seek additional information or clarifications from the report’s author. They will comment on the post with any changes that are made. If a report is deemed to be inappropriate or otherwise requiring deletion, it will be reviewed by a second moderator. If the second moderator concurs, the post may be removed, and the report’s author will be emailed with an explanation of the decision.
If a user enters a case report the data is all entered in a completely de-identified fashion so as not to trigger any patient privacy concerns. Users should be certain that they don’t include any public health information (PHI) in the free-text entry fields. Because the data was collected for the purpose of clinical care and is shared in a deidentified manner, it is not considered research and thus, patient consent is not required.
Interested users who are licensed health care providers complete a very brief registration where they set up an email and password and state their medical qualifications. Once they have registered, a user can now explore the case reports, discussion posts, and clinical trials in the platform; they can submit their own case report or discussion post; or they can view the Newsfeed. Those who can currently enter data are clinicians, physicians, nurses, nurse practitioners, etc.
If you are not a health professional but can contribute to discussions (i.e., as a veterinarian, epidemiologist, infectious disease researcher, etc.) please contact CURE ID or CDRC to find out how you can download and use the app.
The platform is only open to licensed health care professionals so that clinicians will feel comfortable sharing their treatment experiences – both positive and negative – in a safe, clinical community. Registered users have access to all of the data and can contact the help desk for a download of all of the data in the database if they wish to conduct their own research with it.
The data in CURE ID will be digested by CDRC through a dedicated bioinformatic pipeline and the insights generated will be broadly shared with the public via CURE ID once the results have been reviewed and vetted within the Collaboratory.
Shared publicly means that once your data is transformed into the CURE ID format (and it is certain that any PII has been removed), the case report with patient-level data is openly displayed to users who have signed up to use the platform. The information is in addition to the other sources of case reports (electronic health records, registries, literature, etc.).
Yes, the deidentified data as translated into the case report form would be displayed in CURE ID and thus shared with other app users. One can also request to download the data. The intent here is to share what may be working but also what may not be working with other physicians who are desperately looking for ways in which to treat patients in the absence of data.
Yes, we are collecting AEs and have set up a system to automatically share and upload any case reports that contain AEs with the FDA Medwatch AE reporting platform.
No, not at all. The data won’t be combined with data on the same patients or link them, the data will be combined with other sources of case reports, such as registries and clinician-submitted cases to calculate an aggregate of patients treated with the same drug or drug regimens.
Users do not have to sign a data use agreement of any kind (see: case report form for the snapshot of data elements being collected) during the course of routine clinical care and therefore not human subjects research), ensuring that the data transferred is HIPAA compliant and completely de-identified per Safe Harbor requirements, the data can be transferred without a data use agreement. Typically, the individual or institution would translate the patient’s record or EHR/registry data into the CURE ID case report form at their site, before sending the de-identified case report form to the CURE ID platform. The data is then made openly accessible to all qualified clinicians and researchers who register on the site.
At the present time, we are asking contributors to share data broadly which negates the rationale to implement a data use agreement since their data would be shared publicly through the CURE ID platform without restrictions.
Yes, it’s clinical data, but high level – for example: was the patient diagnosed with COVID-19 by PCR? After said diagnosis, what drugs were given to the patient? What was the outcome of treatment (did the patient die, admitted to ICU, discharged from ICU to floor, discharged from hospital, etc. – translated to the options of “patient improved,” “patient deteriorated,” etc.). The CDRC and CURE ID team can assist in creating a translation infrastructure which would then automate the transfer of information from your records to the CURE ID Case Report Form, and then we would upload the case reports and they would be reviewed by clinicians on our staff for quality and accuracy.
Historically, yes, we have collected standardized CURE ID case report forms directly from clinicians and other health care providers using the electronic platform we developed. We have also used this same case report form as an extraction tool to review and enter published case reports into the database. View this as a transformation of your data to populate the simplified CURE ID case report form at your site. An automated system could be used to transform the EHR data at your institutional site into the CURE ID case report form, which would then be sent to NCATS for inclusion on the server.
The ideal scenario would be to use automation to extract the information from the EHRs into the CURE ID template, using a series of rules and translations. However, we realize that that may not be possible. If that is the case, we would be happy to explore using data entry specialists, hired by your . It is also possible, that CURE ID could use a combination of the two – where some data is able to be automatically extracted from the EHRs into CURE ID, and other information requires a human.The development of AI tools will help to translate the data into the case report form, but have human curators review a prespecified portion to ensure that reports are being transformed correctly and that it is of high quality. Some tools have already been developed but will need to be refined to address this specific issue. Collaborators from USG resources and the informatics research community can be accessed to help address the challenges.
No, health care providers are able to enter cases that utilize generic and/or branded drug products. It should be noted, however, that submitting a case report to CURE ID is not a substitute for reporting required under public health or legal requirements. There has been an emphasis on off-patent drugs because they are cheaper to purchase and, in theory, could be easier to access. If a drug, disease, or organism you are interested in reporting a case on is not in the drop-down menu of the app, it can be added by typing the text and then hitting enter.
Off-patent drugs are regulatory-approved medical products that no longer have patent protection. Repurposing approved drugs for new clinical indications can potentially offer an efficient drug-development pathway for treatments of diseases and conditions that have few or no therapeutic options. Advantages of using off-patent drugs in this manner includes cost savings, in that such drugs are often available from a variety of manufacturers. In this same context, however, smaller profit margins may exist for companies that repurpose drugs in the same dosage and formulation for new indications, providing less incentive for repurposing.
No, the focus is on the treatment of this particular infection.
Historically, yes, we have collected standardized CURE ID case report forms directly from clinicians and other health care providers using the electronic platform we developed. We have also used this same case report form as an extraction tool to review and enter published case reports into the database. View this as a transformation of your data to populate the simplified CURE ID case report form at your site. An automated system could be used to transform the EHR data at your institutional site into the CURE ID case report form, which would then be sent to NCATS for inclusion on the server.
The ideal scenario would be to use automation to extract the information from the EHRs into the CURE ID template, using a series of rules and translations. However, we realize that that may not be possible. If that is the case, we would be happy to explore using data entry specialists. It is also possible, that CURE ID could use a combination of the two – where some data is able to be automatically extracted from the EHRs into CURE ID, and other information requires a human. The development of AI tools will help to translate the data into the case report form, but have human curators review a prespecified portion to ensure that reports are being transformed correctly and that it is of high quality. Some tools have already been developed but will need to be refined to address this specific issue. Collaborators from USG resources and the informatics research community can be accessed to help address the challenges.
No, health care providers are able to enter cases that utilize generic and/or branded drug products. It should be noted, however, that submitting a case report to CURE ID is not a substitute for reporting required under public health or legal requirements. There has been an emphasis on off-patent drugs because they are cheaper to purchase and, in theory, could be easier to access. If a drug, disease, or organism you are interested in reporting a case on is not in the drop-down menu of the app, it can be added by typing the text and then hitting enter.
Off-patent drugs are regulatory-approved medical products that no longer have patent protection. Repurposing approved drugs for new clinical indications can potentially offer an efficient drug-development pathway for treatments of diseases and conditions that have few or no therapeutic options. Advantages of using off-patent drugs in this manner includes cost savings, in that such drugs are often available from a variety of manufacturers. In this same context, however, smaller profit margins may exist for companies that repurpose drugs in the same dosage and formulation for new indications, providing less incentive for repurposing.
No, the focus is on the treatment of this particular infection.
For additional questions, please contact, CDRC@c-path.org.

Marco Schito, PhD
Executive Director, CDRC
Smith (Smitty) Heavner, PhD
Senior Scientific Director, CDRC
Jagdeep (JD) Podichetty, PhD
Director, Predictive Analytics
Keith Scollick
Cloud Platform Engineer
Chandler Birch, MS
Senior Project Manager, CDRC
Kitty Bogy
Senior Project Coordinator, CDRC
Pamela Dasher
Senior Project Manager, CDRC
Stacey Coe, MS, CCRP
Senior Clinical Research Coordinator, CDRC
Claire Bassetti, MPH
Communications and Patient Engagement Manager, CDRC
Wes Anderson, PhD
Quantitative Medicine Scientist
Juliette Cnockaert
Dr. Ashima Gupta
For more information on the CURE Drug Repurposing Collaboratory, please email us at cdrc@c-path.org.