Critical Path Institute Announces New Board Members Bonnie A. Allin and Louis Breton
Critical Path Institute (C-Path) today announced the appointments of Bonnie A. Allin and Louis Breton to its Board of Directors.


C-Path’s Predictive Safety Testing Consortium (PSTC) was founded in 2006 to serve as a pre-competitive collaboration for the independent assessment, advancement, and validation of novel drug safety tests.
The tests used to determine drug safety have not changed in decades. Although companies have developed newer methods to test drug safety, these are not globally accepted by the FDA or EMA as proof of safety. This is, in part, because the methods used for testing are often different from company to company. That discrepancy leaves regulatory scientists uncertain about which methods should be preferred. Another key factor is the tests have not, in the past, been independently validated.
PSTC was formed and officially announced by Health and Human Services (HHS) Secretary Michael Leavitt, Food and Drug Administration (FDA) Commissioner Dr. Andrew von Eschenbach, and FDA Deputy Commissioner Dr. Janet Woodcock. Upon its inception, Woodcock described the consortium as “unprecedented” and a “shining example” of the type of work the FDA would like to see conducted.
PSTC’s goal is to obtain regulatory acceptance of novel drug safety tests. PSTC brings together pharmaceutical companies to share and validate innovative safety testing methods under advisement of the U.S. FDA, its European counterpart, the EMA (European Medicines Agency), and PMDA (Japanese Pharmaceutical and Medical Devices Agency). Currently, PSTC is focused on developing and obtaining regulatory qualification of improved clinical safety biomarkers for use in drug development.

Since PSTC’s launch, the consortium achieved qualifications for kidney safety biomarkers from the EMA, PMDA, and FDA for nonclinical use of the biomarkers, summarized in this article. In 2018, the FDA qualified a panel of six kidney safety biomarkers for use in phase one clinical trials. Two recent articles summarize the impact on drug development of the qualified kidney safety biomarkers: Troth et, 2019; Chen et al, 2018. In addition to qualifications, PSTC received several Letters of Support for safety biomarkers to detect drug-induced kidney, skeletal muscle, liver, and vascular injury from the EMA and FDA. Within the consortium, members shared data to support these Qualifications and Letters of Support totaling more than 90 nonclinical studies and more than ten clinical studies.
The kidney safety biomarker Qualifications received by PSTC have enabled more specific and sensitive detection, monitoring, and reversibility of potential kidney injury as described by Dr. Stefan Sultana (AstraZeneca) and Dr. Warren Glaab (Merck & Co., Inc.) during the PSTC 15-year anniversary workshop.
In addition to improving detection and monitoring drug-induced organ injury, where possible, PSTC works with disease-specific consortia across C-Path to provide initial evidence of applicability of the safety biomarkers for future use as diagnostic, prognostic, or efficacy biomarkers.
| Title | Journal | Publication Date | Authors | Link |
|---|---|---|---|---|
| A Panel of Urinary Biomarkers to Monitor Reversibility of Renal Injury and a Serum Marker with Improved Potential to Assess Renal Function | Nature Biotechnology | May, 2010 | Josef S Ozer, Frank Dieterle, Sean Troth, Elias Perentes, André Cordier, Pablo Verdes, Frank Staedtler, Andreas Mahl, Olivier Grenet, Daniel R Roth, Daniel Wahl, François Legay, Daniel Holder, Zoltan Erdos, Katerina Vlasakova, Hong Jin, Yan Yu, Nagaraja Muniappa, Tom Forest, Holly K Clouse, Spencer Reynolds, Wendy J Bailey, Douglas T Thudium, Michael J Topper, Thomas R Skopek, Joseph F Sina, Warren E Glaab, Jacky Vonderscher, Gérard Maurer, Salah-Dine Chibout, Frank D Sistare & David L Gerhold | https://www.ncbi.nlm.nih.gov/pubmed/20458319 |
| A Roadmap for Biomarker Qualification | Nature Biotechnology | May, 2010 | David G Warnock, Carl C Peck | https://www.ncbi.nlm.nih.gov/pubmed/20458313 |
| Brief Overview: Assessment of Compound-induced Acute Kidney Injury Using Animal Models, Biomarkers, and In Vitro Platforms | Toxicologic pathology | November 5, 2018 | James E. McDuffie | https://doi.org/10.1177/0192623318807679 |
| Can Bile Salt Export Pump Inhibition Testing in Drug Discovery and Development Reduce Liver Injury Risk? An International Transporter Consortium Perspective | Clinical Pharmacology & Therapeutics | August 23, 2018 | J. Gerry Kenna, Kunal S. Taskar, Christina Battista, David L. Bourdet, Kim L.R. Brouwer, Kenneth R. Brouwer, David Dai, Christoph Funk, Michael J. Hafey, Yurong Lai, Jonathan Maher, Y. Anne Pak, Jenny M. Pedersen, Joseph W. Polli, A. David Rodrigues, Paul B. Watkins, Kyunghee Yang, Robert W. Yucha | https://doi.org/10.1002/cpt.1222 |
| Candidate biomarkers for the diagnosis and prognosis of drug-induced liver injury: An international collaborative effort | Hepatology | February, 2019 | Rachel J Church, Gerd A Kullak-Ublick, Jiri Aubrecht , Herbert L Bonkovsky, Naga Chalasani, Robert J Fontana, Jens C Goepfert, Frances Hackman, Nicholas M P King, Simon Kirby, Patrick Kirby, John Marcinak, Sif Ormarsdottir, Shelli J Schomaker, Ina Schuppe-Koistinen, Francis Wolenski, Nadir Arber, Michael Merz, John-Michael Sauer, Raul J Andrade, Florian van Bömmel, Thierry Poynard, Paul B Watkins | https://www.ncbi.nlm.nih.gov/pubmed/29357190 |
| Cross-Laboratory Analytical Validation of the Cardiac Biomarker NT-proANP in Rat | Journal of pharmacological and toxicological methods | October 26, 2015 | Petra Vinken, William J Reagan, Luis A Rodriguez, Wayne R Buck, Jie Lai-Zhang, Nick Goeminne, Gaby Barbacci, Ray Liu, Nicholas M P King, Steven K Engle, Heidi Colton | http://www.ncbi.nlm.nih.gov/pubmed/26516096 |
| Current state of cardiac troponin testing in Duchenne muscular dystrophy cardiomyopathy: review and recommendations from the Parent Project Muscular Dystrophy expert panel | Open heart | March 20, 2021 | Christopher F Spurney, Deborah Ascheim, Lawrence Charnas, Linda Cripe, Kan Hor, Nicholas King, Kathi Kinnett, Elizabeth M McNally, John-Michael Sauer, Lee Sweeney, Chet Villa, Larry W Markham | https://pubmed.ncbi.nlm.nih.gov/33762424/ |
| Development and Evaluation of a Genomic Signature for the Prediction and Mechanistic Assessment of Nongenotoxic Hepatocarcinogens in the Rat | Toxicological Sciences | November, 2011 | Mark R Fielden, Alex Adai, Robert T Dunn 2nd, Andrew Olaharski, George Searfoss, Joe Sina, Jiri Aubrecht, Eric Boitier, Paul Nioi, Scott Auerbach, David Jacobson-Kram, Nandini Raghavan, Yi Yang, Andrew Kincaid, Jon Sherlock, Shen-Jue Chen, Bruce Car; Predictive Safety Testing Consortium, Carcinogenicity Working Group | http://www.ncbi.nlm.nih.gov/pubmed/21813463 |
| Evaluation of Cardiac Toxicity Biomarkers in Rats from Different Laboratories | Toxicologic pathology | September 16, 2016 | Kyuri Kim, Naseem Chini, David G Fairchild, Steven K Engle, William J Reagan, Sandra D Summers, Jon C Mirsalis; Cardiac Hypertrophy Working Group of the Predictive Safety Testing Consortium | https://www.ncbi.nlm.nih.gov/pubmed/27638646 |
| Evaluation of Novel Urinary Biomarkers in Beagle Dogs With Amphotericin B-Induced Kidney Injury | International journal of toxicology | March 15, 2023 | Adeyemi O Adedeji, Manisha Sonee, Yafei Chen, Karen Lynch, Katrina Peron, Nicholas King, James E McDuffie, Petra Vinken | https://doi.org/10.1177/10915818221142542 |
| Evaluation of the Relative Performance of Drug-Induced Skeletal Muscle Injury Biomarkers in Rats | Toxicological Sciences | December 31, 2015 | Peter M. Burch, David Greg Hall, Elizabeth G. Walker, William Bracken, Richard Giovanelli, Richard Goldstein, Richard E. Higgs, Nicholas M. P. King, Pamela Lane, John-Michael Sauer, Laura Michna, Nagaraja Muniappa, Michael L. Pritt, Katerina Vlasakova, David E. Watson, Debra Wescott, Tanja S. Zabka, Warren E. Glaab | http://toxsci.oxfordjournals.org/content/early/2015/12/31/toxsci.kfv328 |
| Evolution of Biomarker Qualification at the Health Authorities | Nature biotechnology | May 2, 2010 | Federico Goodsaid, Marisa Papaluca | https://www.ncbi.nlm.nih.gov/pubmed/20458312 |
| Evolving Global Regulatory Science Through the Voluntary Submission of Data: A 2013 Assessment | Therapeutic innovation & regulatory science | October 29, 2013 | Elizabeth Gribble Walker PhD, Martha Brumfield PhD, Carolyn Compton MD, PhD & Raymond Woosley MD, PhD | http://dij.sagepub.com/content/early/2013/10/29/2168479013508941 |
| Immunoaffinity proteomics for kidney injury biomarkers in male beagle dogs | EXCLI Journal | January 3, 2024 | Wael Naboulsi, Hannes Planatscher, Felix F. Schmidt, Andreas Steinhilber, Thomas O. Joos, Adeyemi O. Adedeji, James Eric McDuffie, Oliver Pötz | https://doi.org/10.17179/excli2023-6621 |
| Individual serum bile acid profiling in rats aids in human risk assessment of drug-induced liver injury due to BSEP inhibition | Toxicology and Applied Pharmacology | January 1, 2018 | Steven Cepa, David Potter, Lisa Wong, Leah Schutt, Jacqueline Tarrant, Jodie Pang, Xiaolin Zhang, Roxanne Andaya, Laurent Salphati, Yingqing Ran, Le An, Ryan Morgan, Jonathan Maher | https://doi.org/10.1016/j.taap.2017.11.007 |
| Investigating the Value of Urine Volume, Creatinine, and Cystatin C for Urinary Biomarkers Normalization for Drug Development Studies | International Journal of Toxicology | February 23, 2019 | Adeyemi O Adedeji, Tony Pourmohamad, Yafei Chen, Jennifer Burkey, Catherine J Betts, Susan J Bickerton, Manisha Sonee, James E McDuffie | https://doi.org/10.1177/1091581818819791 |
| Kidney injury Molecule-1 Outperforms Traditional Biomarkers of Kidney Injury in Preclinical Biomarker Qualification Studies | Nature biotechnology | May 11, 2010 | Vishal S Vaidya, Josef S Ozer, Frank Dieterle, Fitz B Collings, Victoria Ramirez, Sean Troth, Nagaraja Muniappa, Douglas Thudium, David Gerhold, Daniel J Holder, Norma A Bobadilla, Estelle Marrer, Elias Perentes, André Cordier, Jacky Vonderscher, Gérard Maurer, Peter L Goering, Frank D Sistare, Joseph V Bonventre | https://www.ncbi.nlm.nih.gov/pubmed/20458318 |
| Molecular biomarkers: a US FDA effort | Biomarkers in medicine | April 2, 2010 | Huixiao Hong, Federico Goodsaid, Leming Shi, Weida Tong | http://www.ncbi.nlm.nih.gov/pubmed/20406066 |
| Next-Generation Biomarkers for Detecting Kidney Toxicity | Nature biotechnology | May 2, 2010 | Joseph V Bonventre, Vishal S Vaidya, Robert Schmouder, Peter Feig, Frank Dieterle | https://www.ncbi.nlm.nih.gov/pubmed/20458311 |
| Nonclinical Safety Biomarkers of Drug-induced Vascular Injury: Current Status and Blueprint for the Future | Toxicologic pathology | April 28, 2014 | Igor Mikaelian, Mark Cameron, Deidre A Dalmas, Bradley E Enerson, Raymond J Gonzalez, Silvia Guionaud, Peter K Hoffmann, Nicholas M P King, Michael P Lawton, Marshall S Scicchitano, Holly W Smith, Roberta A Thomas, James L Weaver, Tanja S Zabka; Vascular Injury Working Group of the Predictive Safety Consortium | http://www.ncbi.nlm.nih.gov/pubmed/24777748 |
| Opportunities and Challenges of Safety Biomarker Qualification: Perspectives from the Predictive Safety Testing Consortium | Drug Development Research | February 22, 2013 | Eslie H. Dennis, Elizabeth G. Walker, Amanda F. Baker, Richard T. Miller | http://onlinelibrary.wiley.com/doi/10.1002/ddr.21070/abstract |
| Performance Assessment of New Urinary Translational Safety Biomarkers of Drug-induced Renal Tubular Injury in Tenofovir-treated Cynomolgus Monkeys and Beagle Dogs | Toxicologic pathology | May 1, 2018 | Yi-Zhong Gu, Katerina Vlasakova, Sean P Troth, Robert L Peiffer, Herve Tournade, Flavia R Pasello Dos Santos, Warren E Glaab, Frank D Sistare | https://doi.org/10.1177/0192623318775023 |
| Perspectives on using a multiplex human kidney safety biomarker panel to detect cisplatin-induced tubular toxicity in male and female Cynomolgus monkeys | Toxicology and Applied Pharmacology | December 1, 2017 | Yafei Chen, J. Dale Thurman, Lewis B. Kinter, Russell Bialecki, J. Eric McDuffie | https://doi.org/10.1016/j.taap.2017.10.010 |
| Preclinical Biomarker Qualification | Experimental biology and medicine | January 1, 2017 | John-Michael Sauer, Amy C Porter; Biomarker Programs, Predictive Safety Testing Consortium | https://www.ncbi.nlm.nih.gov/pubmed/29171289 |
| Qualification of translational safety biomarkers | Experimental biology and medicine | March 19, 2021 | John-Michael Sauer, Amy C Porter | https://pubmed.ncbi.nlm.nih.gov/33757340/ |
| Renal Biomarker Qualification Submission: A Dialog Between the FDA-EMEA and Predictive Safety Testing Consortium | Nature biotechnology | May 11, 2010 | Frank Dieterle, Frank Sistare, Federico Goodsaid, Marisa Papaluca, Josef S Ozer, Craig P Webb, William Baer, Anthony Senagore, Matthew J Schipper, Jacky Vonderscher, Stefan Sultana, David L Gerhold, Jonathan A Phillips, Gérard Maurer, Kevin Carl, David Laurie, Ernie Harpur, Manisha Sonee, Daniela Ennulat, Dan Holder, Dina Andrews-Cleavenger, Yi-Zhong Gu, Karol L Thompson, Peter L Goering, Jean-Marc Vidal, Eric Abadie, Romaldas Maciulaitis, David Jacobson-Kram, Albert F Defelice, Elizabeth A Hausner, Melanie Blank, Aliza Thompson, Patricia Harlow, Douglas Throckmorton, Shen Xiao, Nancy Xu, William Taylor, Spiros Vamvakas, Bruno Flamion, Beatriz Silva Lima, Peter Kasper, Markku Pasanen, Krishna Prasad, Sean Troth, Denise Bounous, Denise Robinson-Gravatt, Graham Betton, Myrtle A Davis, Jackie Akunda, James Eric McDuffie, Laura Suter, Leslie Obert, Magalie Guffroy, Mark Pinches, Supriya Jayadev, Eric A Blomme, Sven A Beushausen, Valérie G Barlow, Nathaniel Collins, Jeff Waring, David Honor, Sandra Snook, Jinhe Lee, Phil Rossi, Elizabeth Walker, William Mattes | http://www.ncbi.nlm.nih.gov/pubmed/20458315 |
| Research at the Interface of Industry, Academia and Regulatory Science | Nature biotechnology | May 2, 2010 | William B Mattes, Elizabeth Gribble Walker, Eric Abadie, Frank D Sistare, Jacky Vonderscher, Janet Woodcock, Raymond L Woosley | http://www.ncbi.nlm.nih.gov/pubmed/20458309 |
| Response of Novel Skeletal Muscle Biomarkers in Dogs to Drug-Induced Skeletal Muscle Injury or Sustained Endurance Exercise | Toxicological Sciences | April 1, 2017 | Katerina Vlasakova, Pamela Lane, Laura Michna, Nagaraja Muniappa, Frank D. Sistare, Warren E. Glaab | https://doi.org/10.1093/toxsci/kfw262 |
| Scientific and Regulatory Considerations for the Analytical Validation of Assays Used in the Qualification of Biomarkers in Biological Matrices | June 11, 2019 | Steven P. Piccoli, GlaxoSmithKline and John Michael Sauer, Critical Path Institute | https://media.c-path.org/wp-content/uploads/20240427170640/EvidConsid-WhitePaper-AnalyticalSectionV20190621.pdf | |
| Serum Glutamate Dehydrogenase Activity Enables Early Detection of Liver Injury in Subjects with Underlying Muscle Impairments | PloS one | May 14, 2020 | Shelli Schomaker, David Potter, Roscoe Warner, Jane Larkindale, Nicholas King, Amy C Porter, Jane Owens, Lindsay Tomlinson, John-Michael Sauer, Kent Johnson, Jiri Aubrecht | https://pubmed.ncbi.nlm.nih.gov/32407333/ |
| Strategic Paths for Biomarker Qualification | Toxicology | March 21, 2008 | Federico M Goodsaid,, Felix W Frueh, William Mattes | http://www.ncbi.nlm.nih.gov/pubmed/18280028 |
| The Predictive Safety Testing Consortium And The Coalition Against Major Diseases | Nature reviews. Drug discovery | November 1, 2014 | Diane Stephenson, John-Michael Sauer | http://www.ncbi.nlm.nih.gov/pubmed/25359364 |
| The Predictive Safety Testing Consortium: Safety Biomarkers, Collaboration, and Qualification | Journal of Medicines Development Sciences | July 14, 2015 | John-Michael Sauer , Elizabeth G Walker and Amy C Porter | https://c-path.org//wp-content/uploads/2015/11/The-Predictive-Safety-Testing-Consortium_safety-bio-markers-collaboration-and-qualification_Jun2015.pdf |
| The Utility of Novel Urinary Biomarkers in Mice for Drug Development Studies | International journal of toxicology | January 19, 2021 | Adeyemi O Adedeji, Yi-Zhong Gu, Tony Pourmohamad, Justin Kanerva, Yafei Chen, Elnaz Atabakhsh, Michael R Tackett, Feifei Chen, Bhavana Bhatt, Thierry Gury, Olivier Dorchies, Manisha Sonee, Michelle Morgan, Jennifer Burkey, Jean-Charles Gautier, James E McDuffie | http://www.ncbi.nlm.nih.gov/pubmed/33161787/ |
| Towards Consensus Practices to Qualify Safety Biomarkers for Use in Early Drug Development | Nature biotechnology | May 11, 2010 | Frank D Sistare, Frank Dieterle, Sean Troth, Daniel J Holder, David Gerhold, Dina Andrews-Cleavenger, William Baer, Graham Betton, Denise Bounous, Kevin Carl, Nathaniel Collins, Peter Goering, Federico Goodsaid, Yi-Zhong Gu, Valerie Guilpin, Ernie Harpur, Alita Hassan, David Jacobson-Kram, Peter Kasper, David Laurie, Beatriz Silva Lima, Romaldas Maciulaitis, William Mattes, Gérard Maurer, Leslie Ann Obert, Josef Ozer, Marisa Papaluca-Amati, Jonathan A Phillips, Mark Pinches, Matthew J Schipper, Karol L Thompson, Spiros Vamvakas, Jean-Marc Vidal, Jacky Vonderscher, Elizabeth Walker, Craig Webb, Yan Yu | http://www.ncbi.nlm.nih.gov/pubmed/20458314 |
| Translational Medicine and the Value of Biomarker Qualification | Science translational medicine | September 2, 2010 | Federico M Goodsaid, Donna L Mendrick | http://www.ncbi.nlm.nih.gov/pubmed/20811041 |
| Translational Toxicology and the Work of the Predictive Safety Testing Consortium | Clinical pharmacology and therapeutics | January 21, 2009 | W B Mattes, E G Walker | http://www.ncbi.nlm.nih.gov/pubmed/19158666 |
| Urinary Biomarkers Trefoil Factor 3 and Albumin Enable Early Detection of Kidney Tubular Injury | Nature biotechnology | May 11, 2010 | Yan Yu, Hong Jin, Daniel Holder, Josef S Ozer, Stephanie Villarreal, Paul Shughrue, Shu Shi, David J Figueroa, Holly Clouse, Ming Su, Nagaraja Muniappa, Sean P Troth, Wendy Bailey, John Seng, Amy G Aslamkhan, Douglas Thudium, Frank D Sistare, David L Gerhold | https://www.ncbi.nlm.nih.gov/pubmed/20458317 |
| Urinary Clusterin, Cystatin C, [beta]2-Microglobulin and Total Protein as Markers to Detect Drug-Induced Kidney Injury | Nature biotechnology | May 11, 2010 | Frank Dieterle, Elias Perentes, André Cordier, Daniel R Roth, Pablo Verdes, Olivier Grenet, Serafino Pantano, Pierre Moulin, Daniel Wahl, Andreas Mahl, Peter End, Frank Staedtler, François Legay, Kevin Carl, David Laurie, Salah-Dine Chibout, Jacky Vonderscher, Gérard Maurer | https://www.ncbi.nlm.nih.gov/pubmed/20458316 |
| What Evidence Do We Need for Biomarker Qualification? | Science translational medicine | November 22, 2017 | Chris Leptak, Joseph P Menetski, John A Wagner, Jiri Aubrecht, Linda Brady, Martha Brumfield, William W Chin, Steve Hoffmann, Gary Kelloff, Gabriela Lavezzari, Rajesh Ranganathan, John-Michael Sauer, Frank D Sistare, Tanja Zabka, David Wholley | https://www.ncbi.nlm.nih.gov/pubmed/29167393 |
The US Food and Drug Administration (FDA) issued its first ever qualification of a clinical safety biomarker based on data submitted jointly by the Foundation for the National Institutes of Health (FNIH) Biomarkers Consortium (BC) and the Critical Path Institute’s (C-Path) Predictive Safety Testing Consortium (PSTC).
The qualification applies to a single composite measure (CM) of six urine biomarkers, to be used in conjunction with traditional measures of kidney function; the six safety biomarkers include clusterin (CLU), cystatin-C (CysC), kidney injury molecule-1 (KIM-1), N-acetyl-beta-D-glucosaminidase (NAG), neutrophil gelatinase-associated lipocalin (NGAL), and osteopontin (OPN).
The purpose and Context of Use (COU) for this qualified biomarker are as follows: A safety composite biomarker panel to be used in conjunction with traditional measures to aid in the detection of kidney tubular injury in phase 1 trials in healthy volunteers when there is an a priori concern that a drug may cause renal tubular injury in humans.
Use of the CM biomarker will help improve the development of safe and effective medicines where concern has been raised that an investigational drug may cause kidney injury. As stated in the FDA’s Qualification Decision and Executive Summary, “This biomarker can be used by drug developers for the qualified COU in submissions of investigational new drug applications (INDs), new drug applications (NDAs), and biologics license applications (BLAs) without the need to resubmit the biomarker information or rereview by the relevant [Center for Drug Evaluation and Research] CDER disciplines.”
The CM is qualified as a safety biomarker for dose cohorts, not for individual patient/study subject monitoring.
Using the CM in Phase 1 clinical trials (with healthy volunteers as subjects) in conjunction with standard measures of renal function (serum creatinine [sCr], blood urea nitrogen [BUN], urine albumin, and urine total protein) can help clinicians and investigators monitor the risk of renal tubular injury and may inform subsequent decision-making in the drug development process.
Additional details are available in the User’s Guide: Kidney Safety Composite Measure Biomarker for Use in Clinical Development.
Decision tree for clinical use of qualified CM biomarker in a Phase 1 trial involving healthy human subjects

Included with the qualification determination are important considerations and sensible practices related to use of the CM; these are outlined in the FDA’s Qualification Decision and Executive Summary.
Additional details regarding this biomarker qualification, including specific information about how it can be used in drug development programs and clinical trials, are available on the FDA’s Biomarker Qualification Program website.
Following several years of PSTC’s collaborative research, the FDA, European Medicines Agency (EMA), and the Japanese Pharmaceuticals and Medical Devices Agency (PMDA) have each qualified seven novel laboratory tests on urine which signal kidney injury. The safety biomarkers were evaluated in data from rat studies submitted to the regulatory agencies by PSTC. The qualified biomarkers are kidney injury molecule-1, albumin, total protein, β2-microglobulin, cystatin C, clusterin, and trefoil factor-3.
These tests can be used in laboratory research to predict the safety of experimental drugs. The details of this research and qualification are available in these articles:
http://www.ncbi.nlm.nih.gov/sites/myncbi/collections/public/1h9C-otw1T5zb8Ra6o0EUYzkG/
The FDA has issued a Letter of Support to the Predictive Safety Testing Consortium (PSTC) to encourage the further study and use of a set of translational safety biomarkers to detect and monitor drug-induced pancreatic injury (DIPI). The set of biomarkers are four micro RNAs (miRNAs) under investigation for DIPI are miR-216a, miR-216b, miR-217, and miR-375.
The translational pancreatic safety biomarkers (miR-216a, miR-216b, miR-217, and miR-375) will be used in conjunction with amylase and lipase to aid in the detection of DIPI in Phase 1 clinical trials when there is demonstrated monitorability of induced damage in nonclinical studies and sufficient safety margins or when there is a priori concern that a drug candidate may induce DIPI due to known structural class similarities or identified target liability concerns.
Novel biomarkers of DIPI used in combination with currently available biomarkers could play an important role in guiding safety assessment in phase I clinical trials. Although the standard markers of pancreas injury, amylase and lipase, are currently the clinically accepted biomarkers for the diagnosis of acute pancreatitis (IAP/APA evidence-based guidelines for the management of acute pancreatitis, 2013), it is widely acknowledged that they are not sufficiently sensitive nor specific to guide dosing-related decisions in clinical trials (Lee and Papachristou, 2019; Ismail and Bhayana, 2017).
We support PSTC’s initiative to encourage the voluntary and complementary use of these miRNAs in conjunction with amylase and lipase as exploratory nonclinical and clinical biomarkers of DIPI. We also support PSTC’s generation of additional nonclinical toxicology data and plan for exploratory early clinical studies to enable future formal qualification of these safety biomarkers.
MiRNA molecules are short non-coding sequences ranging from 19 to 25 nucleotides in length, which are involved mainly in the post-transcriptional modulation of gene expression. A diverse array of functions including cellular signaling, cell growth and differentiation and apoptosis have been described for miRNAs. In humans, approximately 2,600 miRNA genes are currently known. Information on miRNA identification and nomenclature can be found at www.mirbase.org. Because of the relative abundance of miRNAs in specific tissues, they are increasingly being recognized as leakage biomarkers indicative of tissue injury (Bailey and Glaab, 2018; Schraml et al., 2017). A number of candidate miRNAs have been identified and studied in association with pancreatic acinar injury in nonclinical species (Erdos, 2020; Usborne, 2014), including miR-217, miR-216a, miR-216b, and miR-375.
Member companies of the PSTC PIWG have conducted studies and assessed pancreas-specific miRNAs to determine their performance in monitoring drug-induced toxicities. Toxicants targeting Exocrine/Acinar Predominant Injury (caerulein, caerulein perfusion, cholecystokinin, L-arginine, cyanohydroxybutene, DL-Ethionine, sodium taurocholate, Dibutyltin dichloride, Ethanol + Cyclosporin A + caerulein, ductal retrograde trinitrobenzene sulfonic acid, and proprietary drug candidates) and Endocrine/Islet Predominant Injury (streptozotocin and proprietary drug candidates) assessed the performance of the miRNAs to detect DIPI in rats (all toxicants) and dog (only cholecystokinin). The studies supporting this Letter of Support were published in peerreviewed scientific journals. These pancreas-specific miRNAs have been evaluated as biomarkers of DIPI and show promise to add value to the interpretation of amylase and lipase in monitoring acute pancreatic injury defined as acinar cell degeneration/necrosis.
More experience with the use of this biomarker in clinical studies would be useful to determine its utility more accurately for detecting and monitoring drug-induced pancreatic injury. MicroRNA thresholds have yet to be determined in clinical samples. Assessment will be in conjunction with amylase and lipase and not be used independently for decision making. Future studies will determine the clinical thresholds and the interpretation of results including performance metrics. We encourage exploration of these miRNAs (miR-217, miR-216a, miR-216b, and miR-375) used, in conjunction with amylase and lipase, to aid in the detection of DIPI in Phase 1 clinical trials when there is demonstrated monitorability of induced damage in nonclinical studies and sufficient safety margins or when there is a prior concern that a drug candidate may induce DIPI due to known structural class similarities or identified target liability concerns.
Any groups (academia, industry, government) that would like to join in this effort or have information or data that may be useful can contact Mr. Nicholas King (nking@c-path.org) or view the PSTC website.
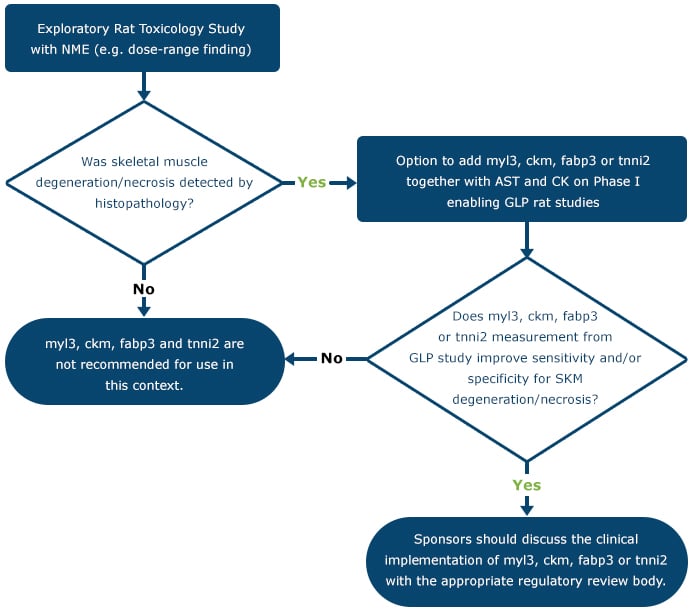
The U.S. Food and Drug Administration (FDA) and European Medicines Agency, recently, issued Biomarker Letters of Support (LOS) for the skeletal muscle injury safety biomarkers myosin light chain 3 (Myl3) and skeletal muscle troponin I (sTnI), fatty acid binding protein 3 (Fabp3), and creatine kinase M (CK-M) based on data submitted by the Critical Path Institute’s (C-Path) Predictive Safety Testing Consortium’s (PSTC) Skeletal Myopathy Working Group (SKMWG). These letters briefly describe the U.S. FDA’s and EMA’s thoughts on the value of Myl3, sTnI, Fabp3, and Ckm and encourage further evaluation of biomarkers with promising utility in drug development.
The Summary Data Package contains summary statistics, description of analysis methods, and results tables submitted to the EMA and U.S. FDA for the regulatory decision on use of serum/plasma Myl3, sTnI, Fabp3, and Ckm in nonclinical and exploratory clinical studies.
Decision tree for translational use of Myl3, sTnI, FABP3, and CK-M in a drug development program
Following the determination that Myl3, sTnI, Fabp3, and Ckm can add value to conventional biomarkers for monitoring skeletal muscle degeneration/necrosis in a prospective rat GLP study, sponsors are encouraged to discuss proposals for use of human Myl3, sTnI, Fabp3, and Ckm to enable the safe conduct of clinical studies with the appropriate regulatory review body.
The U.S. Food and Drug Administration (FDA) and European Medicines Agency (EMA), recently, issued Biomarker Letters of Support (LOS) for the drug-induced vascular injury safety biomarkers for the following: endothelial cell proteins (E‐Selectin, P‐Selectin, sICAM‐1, sICAM‐3, sVCAM‐1, thrombomodulin, and VEGF); smooth muscle cell proteins (EMA only: calponin, caldesmon); and inflammatory factors (CRP, GROa, NGAL, IL‐6, IL‐8, IP‐10, I‐TAC, MCP‐1, MIG, SAA, MIP‐1α, and TIMP-1) based on data submitted by the Innovative Medicine Initiative’s (IMI) Safer and Faster Evidence-based Translation Consortium (SAFE-T) and the Critical Path Institute’s (C-Path) Predictive Safety Testing Consortium’s (PSTC) Vascular Injury Working Group (VIWG).
These letters, describe the U.S. FDA’s and EMA’s thoughts on the value of the DIVI biomarkers and encourages further evaluation. The EMA’s LOS encourages “further nonclinical and exploratory clinical analyses to evaluate the translational relevance of changes in identified nonclinical DIVI biomarkers.” The FDA’s LOS encourages “additional use of these biomarkers to characterize their potential role for monitoring DIVI in early clinical drug development.”
Although not qualified by FDA or EMA, these translatable biomarkers, alone or in panel(s), are anticipated to reflect DIVI affecting vascular smooth muscle cells and endothelial cells, as well as the associated inflammatory response, as determined by histopathologic endpoints in rodents. It is envisioned that the vascular injury biomarkers will ultimately be used in healthy volunteers with no concurrent vascular disease to monitor for vascular safety in early clinical trials. These biomarkers will be used when such injury has been demonstrated to be monitorable by translatable biomarkers in animal studies of similar duration with the same test agent (including small and large molecule therapeutics). Applying the biomarkers in initial single and multiple ascending dose clinical studies could help inform planned dose escalations or continued dosing schedules.
The Summary Data Package contains summary statistics, description of analysis methods, and results tables submitted to the EMA and U.S. FDA for the regulatory decision on use of the DIVI biomarkers in nonclinical and exploratory clinical studies.
The U.S. Food and Drug Administration (FDA) and European Medicines Agency, recently, issued Biomarker Letters of Support (LOS) for the kidney safety biomarkers osteopontin (OPN) and neutrophil gelatinase-associated lipocalin (NGAL) based on data submitted by the Critical Path Institute’s (C-Path) Predictive Safety Testing Consortium’s (PSTC) Nephrotoxicity Working Group (NWG). The Summary Data Package reports the relationship between kidney proximal tubule degeneration/necrosis and urinary levels of OPN and NGAL in rodents. These letters briefly describe the U.S. FDA’s and EMA’s thoughts on the value of urinary OPN and NGAL and encourage further evaluation of biomarkers with promising utility in drug development.
The Summary Data Package contains summary statistics, description of analysis methods, and results tables submitted to the EMA and U.S. FDA for the regulatory decision on use of urinary OPN and NGAL in nonclinical and exploratory clinical studies.
Following the determination that OPN and NGAL can add value to conventional biomarkers for monitoring kidney tubular degeneration/necrosis in a prospective rat GLP study, sponsors are encouraged to discuss proposals for use of human urinary OPN and NGAL to enable the safe conduct of clinical studies with the appropriate regulatory review body.

The European Medicines Agency (EMA) and United States Food and Drug Administration (FDA) have both issued separate Letters of Support (LOS) to encourage the further development and exploratory use of percent change from baseline of the following urinary markers: alpha-glutathione S-transferase (α-GST), clusterin (CLU), cystatin C (CysC), kidney injury molecule-1 (KIM-1), neutrophil gelatinase-associated lipocalin (NGAL), osteopontin (OPN), albumin (ALB) and total protein (TPRO) as biomarkers of drug-induced renal tubular injury in early clinical trials. The EMA also supports the use of serum cystatin C utilizing the percent change from baseline approach proposed by the DIKI group. These Letters of Support are based on data submitted by the Innovative Medicine Initiative’s (IMI) Safer and Faster Evidence-based Translation Consortium (SAFE-T) and the Critical Path Institute’s (C-Path) Predictive Safety Testing Consortium’s (PSTC) Nephrotoxicity Working Group (HWG).
These letters describe FDA’s and EMA’s thoughts on the value of α-GST, CLU, CysC, KIM-1, NGAL, OPN, ALB and TPRO and encourages further evaluation. The FDA’s LOS encourages “the exploratory use of these urinary biomarkers (α-GST, CLU, CysC, KIM-1, NGAL, OPN, ALB, and TPRO) as biomarkers of renal tubule injury in early clinical trials.”
Additionally, the FDA stated that the “exploratory human data suggests that the candidate drug-induced renal tubular injury biomarkers may be more sensitive and specific for the detection of acute kidney injury, especially when used in combination, than traditional means of monitoring for nephrotoxicity. In addition, you observed a rise in most of the candidate biomarkers (urinary a-GST, CLU, KIM-1, NGAL, CysC, and OPN) that preceded a clinically-relevant rise in sCr.”
The Summary Data Package contains summary statistics, description of analysis methods, and results tables submitted to the EMA and U.S. FDA.
The European Medicines Agency (EMA), recently, issued a Biomarker LOS, for the drug-induced liver injury safety biomarkers Glutamate Dehydrogenase (GLDH) based on data submitted by the Critical Path Institute’s (C-Path) Predictive Safety Testing Consortium’s (PSTC) Hepatotoxicity Working Group (HWG) and the Duchenne Regulatory Science Consortium (D-RSC).
This letter briefly describes EMA’s thoughts on the value of GLDH and encourages further evaluation. The EMA’s LOS supports “PSTC’s and D-RSC’s initiative to encourage investigation of the voluntary and complementary use of serum GLDH, in conjunction with currently used biomarkers of liver injury, as a clinical biomarker of liver injury. The Agency also supports PSTC’s generation of additional clinical safety data and plans for further clinical studies to potentially enable formal qualification of GLDH in the future.”
The U.S. Food and Drug Administration (FDA) issued Biomarker Letters of Support for the drug-induced liver injury safety biomarkers Cytokeratin 18 (CK-18), Total and hyperacetylated high mobility group protein B1 (HMGB1), Osteopontin, and Macrophage colony-stimulating factor 1 receptor (CSF1R) based on data submitted by the Innovative Medicine Initiative’s (IMI) Safer and Faster Evidence-based Translation Consortium (SAFE-T) and the Critical Path Institute’s (C-Path) Predictive Safety Testing Consortium’s (PSTC) Hepatotoxicity Working Group (HWG).
This letter briefly describes U.S. FDA’s thoughts on the value of CK-18, HMGB1, Osteopontin, and CSF1R and encourages further evaluation. The U.S. FDA’s LOS encourages further evaluation of biomarkers with promising utility in drug development either “alone or in combination as soluble monitoring biomarkers to assess the risk of progression of drug-induced liver injury (DILI) in patients in whom an initial DILI diagnosis has been established based on elevations of the standard biomarkers alanine aminotransferase (ALT) alone or in combination with total bilirubin (TBIL) as a clinical safety assessment in clinical trials in a drug development context.”
In concert with C-Path’s Duchenne Regulatory Science Consortium, on November 8, 2019 PSTC submitted a Letter of Intent (LOI) to the U.S Food and Drug Administration (FDA) for a panel of four novel safety biomarkers of acute drug-induced muscle injury (DIMI) in phase I clinical trials with healthy volunteers. The LOI received a positive response and has been accepted into FDA’s Center for Drug Evaluation and Research (CDER) Biomarker Qualification Program (BQP).
Click here to read the press release and view the Acceptance Letter.
PSTC and D-RSC have been invited to submit a Qualification Plan (QP) for the DIMI biomarker panel, the second stage in the BQP process that details additional clinical studies and analysis for the intended Context of Use.
In concert with C-Path’s Duchenne Regulatory Science Consortium (D-RSC), on August 23, 2019 PSTC submitted a Qualification Plan (QP) to the U.S Food and Drug Administration (FDA) for glutamate dehydrogenase (GLDH) as a safety biomarker for detecting drug-induced liver injury (DILI) in clinical trials involving patients affected by inherited muscle disorders as well as muscle damage caused by strenuous exercise and drug-induced muscle injury. The submission received a positive response and has been accepted to FDA’s Center for Drug Evaluation and Research (CDER) Biomarker Qualification Program (BQP).
Click here to read the press release and view the Qualification Plan Determination.
PSTC and D-RSC have been invited to submit a Full Qualification Package, the final stage of qualification, that demonstrates clinical and analytical validity for the biomarker’s intended Context of Use.
The FDA has issued a Letter of Support to the Predictive Safety Testing Consortium (PSTC) to encourage the further study and use of a set of translational safety biomarkers to detect and monitor drug-induced pancreatic injury (DIPI). The set of biomarkers are four micro RNAs (miRNAs) under investigation for DIPI are miR-216a, miR-216b, miR-217, and miR-375.
The translational pancreatic safety biomarkers (miR-216a, miR-216b, miR-217, and miR-375) will be used in conjunction with amylase and lipase to aid in the detection of DIPI in Phase 1 clinical trials when there is demonstrated monitorability of induced damage in nonclinical studies and sufficient safety margins or when there is a priori concern that a drug candidate may induce DIPI due to known structural class similarities or identified target liability concerns.
Novel biomarkers of DIPI used in combination with currently available biomarkers could play an important role in guiding safety assessment in phase I clinical trials. Although the standard markers of pancreas injury, amylase and lipase, are currently the clinically accepted biomarkers for the diagnosis of acute pancreatitis (IAP/APA evidence-based guidelines for the management of acute pancreatitis, 2013), it is widely acknowledged that they are not sufficiently sensitive nor specific to guide dosing-related decisions in clinical trials (Lee and Papachristou, 2019; Ismail and Bhayana, 2017).
We support PSTC’s initiative to encourage the voluntary and complementary use of these miRNAs in conjunction with amylase and lipase as exploratory nonclinical and clinical biomarkers of DIPI. We also support PSTC’s generation of additional nonclinical toxicology data and plan for exploratory early clinical studies to enable future formal qualification of these safety biomarkers.
MiRNA molecules are short non-coding sequences ranging from 19 to 25 nucleotides in length, which are involved mainly in the post-transcriptional modulation of gene expression. A diverse array of functions including cellular signaling, cell growth and differentiation and apoptosis have been described for miRNAs. In humans, approximately 2,600 miRNA genes are currently known. Information on miRNA identification and nomenclature can be found at www.mirbase.org. Because of the relative abundance of miRNAs in specific tissues, they are increasingly being recognized as leakage biomarkers indicative of tissue injury (Bailey and Glaab, 2018; Schraml et al., 2017). A number of candidate miRNAs have been identified and studied in association with pancreatic acinar injury in nonclinical species (Erdos, 2020; Usborne, 2014), including miR-217, miR-216a, miR-216b, and miR-375.
Member companies of the PSTC PIWG have conducted studies and assessed pancreas-specific miRNAs to determine their performance in monitoring drug-induced toxicities. Toxicants targeting Exocrine/Acinar Predominant Injury (caerulein, caerulein perfusion, cholecystokinin, L-arginine, cyanohydroxybutene, DL-Ethionine, sodium taurocholate, Dibutyltin dichloride, Ethanol + Cyclosporin A + caerulein, ductal retrograde trinitrobenzene sulfonic acid, and proprietary drug candidates) and Endocrine/Islet Predominant Injury (streptozotocin and proprietary drug candidates) assessed the performance of the miRNAs to detect DIPI in rats (all toxicants) and dog (only cholecystokinin). The studies supporting this Letter of Support were published in peerreviewed scientific journals. These pancreas-specific miRNAs have been evaluated as biomarkers of DIPI and show promise to add value to the interpretation of amylase and lipase in monitoring acute pancreatic injury defined as acinar cell degeneration/necrosis.
More experience with the use of this biomarker in clinical studies would be useful to determine its utility more accurately for detecting and monitoring drug-induced pancreatic injury. MicroRNA thresholds have yet to be determined in clinical samples. Assessment will be in conjunction with amylase and lipase and not be used independently for decision making. Future studies will determine the clinical thresholds and the interpretation of results including performance metrics. We encourage exploration of these miRNAs (miR-217, miR-216a, miR-216b, and miR-375) used, in conjunction with amylase and lipase, to aid in the detection of DIPI in Phase 1 clinical trials when there is demonstrated monitorability of induced damage in nonclinical studies and sufficient safety margins or when there is a prior concern that a drug candidate may induce DIPI due to known structural class similarities or identified target liability concerns.
Any groups (academia, industry, government) that would like to join in this effort or have information or data that may be useful can contact Mr. Nicholas King (nking@c-path.org) or view the PSTC website.
The U.S. Food and Drug Administration (FDA) and European Medicines Agency, recently, issued Biomarker Letters of Support (LOS) for the skeletal muscle injury safety biomarkers myosin light chain 3 (Myl3) and skeletal muscle troponin I (sTnI), fatty acid binding protein 3 (Fabp3), and creatine kinase M (CK-M) based on data submitted by the Critical Path Institute’s (C-Path) Predictive Safety Testing Consortium’s (PSTC) Skeletal Myopathy Working Group (SKMWG). These letters briefly describe the U.S. FDA’s and EMA’s thoughts on the value of Myl3, sTnI, Fabp3, and Ckm and encourage further evaluation of biomarkers with promising utility in drug development.
The Summary Data Package contains summary statistics, description of analysis methods, and results tables submitted to the EMA and U.S. FDA for the regulatory decision on use of serum/plasma Myl3, sTnI, Fabp3, and Ckm in nonclinical and exploratory clinical studies.
Decision tree for translational use of Myl3, sTnI, FABP3, and CK-M in a drug development program
Following the determination that Myl3, sTnI, Fabp3, and Ckm can add value to conventional biomarkers for monitoring skeletal muscle degeneration/necrosis in a prospective rat GLP study, sponsors are encouraged to discuss proposals for use of human Myl3, sTnI, Fabp3, and Ckm to enable the safe conduct of clinical studies with the appropriate regulatory review body.

The U.S. Food and Drug Administration (FDA) and European Medicines Agency (EMA), recently, issued Biomarker Letters of Support (LOS) for the drug-induced vascular injury safety biomarkers for the following: endothelial cell proteins (E‐Selectin, P‐Selectin, sICAM‐1, sICAM‐3, sVCAM‐1, thrombomodulin, and VEGF); smooth muscle cell proteins (EMA only: calponin, caldesmon); and inflammatory factors (CRP, GROa, NGAL, IL‐6, IL‐8, IP‐10, I‐TAC, MCP‐1, MIG, SAA, MIP‐1α, and TIMP-1) based on data submitted by the Innovative Medicine Initiative’s (IMI) Safer and Faster Evidence-based Translation Consortium (SAFE-T) and the Critical Path Institute’s (C-Path) Predictive Safety Testing Consortium’s (PSTC) Vascular Injury Working Group (VIWG).
These letters, describe the U.S. FDA’s and EMA’s thoughts on the value of the DIVI biomarkers and encourages further evaluation. The EMA’s LOS encourages “further nonclinical and exploratory clinical analyses to evaluate the translational relevance of changes in identified nonclinical DIVI biomarkers.” The FDA’s LOS encourages “additional use of these biomarkers to characterize their potential role for monitoring DIVI in early clinical drug development.”
Although not qualified by FDA or EMA, these translatable biomarkers, alone or in panel(s), are anticipated to reflect DIVI affecting vascular smooth muscle cells and endothelial cells, as well as the associated inflammatory response, as determined by histopathologic endpoints in rodents. It is envisioned that the vascular injury biomarkers will ultimately be used in healthy volunteers with no concurrent vascular disease to monitor for vascular safety in early clinical trials. These biomarkers will be used when such injury has been demonstrated to be monitorable by translatable biomarkers in animal studies of similar duration with the same test agent (including small and large molecule therapeutics). Applying the biomarkers in initial single and multiple ascending dose clinical studies could help inform planned dose escalations or continued dosing schedules.
The Summary Data Package contains summary statistics, description of analysis methods, and results tables submitted to the EMA and U.S. FDA for the regulatory decision on use of the DIVI biomarkers in nonclinical and exploratory clinical studies.
The U.S. Food and Drug Administration (FDA) and European Medicines Agency, recently, issued Biomarker Letters of Support (LOS) for the kidney safety biomarkers osteopontin (OPN) and neutrophil gelatinase-associated lipocalin (NGAL) based on data submitted by the Critical Path Institute’s (C-Path) Predictive Safety Testing Consortium’s (PSTC) Nephrotoxicity Working Group (NWG).
The Summary Data Package reports the relationship between kidney proximal tubule degeneration/necrosis and urinary levels of OPN and NGAL in rodents. These letters briefly describe the U.S. FDA’s and EMA’s thoughts on the value of urinary OPN and NGAL and encourage further evaluation of biomarkers with promising utility in drug development.
The Summary Data Package contains summary statistics, description of analysis methods, and results tables submitted to the EMA and U.S. FDA for the regulatory decision on use of urinary OPN and NGAL in nonclinical and exploratory clinical studies.
Following the determination that OPN and NGAL can add value to conventional biomarkers for monitoring kidney tubular degeneration/necrosis in a prospective rat GLP study, sponsors are encouraged to discuss proposals for use of human urinary OPN and NGAL to enable the safe conduct of clinical studies with the appropriate regulatory review body.

The European Medicines Agency (EMA), recently, issued a Biomarker LOS, for the drug-induced liver injury safety biomarkers Glutamate Dehydrogenase (GLDH) based on data submitted by the Critical Path Institute’s (C-Path) Predictive Safety Testing Consortium’s (PSTC) Hepatotoxicity Working Group (HWG) and the Duchenne Regulatory Science Consortium (D-RSC).
This letter briefly describes EMA’s thoughts on the value of GLDH and encourages further evaluation. The EMA’s LOS supports “PSTC’s and D-RSC’s initiative to encourage investigation of the voluntary and complementary use of serum GLDH, in conjunction with currently used biomarkers of liver injury, as a clinical biomarker of liver injury. The Agency also supports PSTC’s generation of additional clinical safety data and plans for further clinical studies to potentially enable formal qualification of GLDH in the future.”
Decision tree for clinical use of GLDH

The U.S. Food and Drug Administration (FDA) issued Biomarker Letters of Support for the drug-induced liver injury safety biomarkers Cytokeratin 18 (CK-18), Total and hyperacetylated high mobility group protein B1 (HMGB1), Osteopontin, and Macrophage colony-stimulating factor 1 receptor (CSF1R) based on data submitted by the Innovative Medicine Initiative’s (IMI) Safer and Faster Evidence-based Translation Consortium (SAFE-T) and the Critical Path Institute’s (C-Path) Predictive Safety Testing Consortium’s (PSTC) Hepatotoxicity Working Group (HWG).
This letter briefly describes U.S. FDA’s thoughts on the value of CK-18, HMGB1, Osteopontin, and CSF1R and encourages further evaluation. The U.S. FDA’s LOS encourages further evaluation of biomarkers with promising utility in drug development either “alone or in combination as soluble monitoring biomarkers to assess the risk of progression of drug-induced liver injury (DILI) in patients in whom an initial DILI diagnosis has been established based on elevations of the standard biomarkers alanine aminotransferase (ALT) alone or in combination with total bilirubin (TBIL) as a clinical safety assessment in clinical trials in a drug development context.”
The European Medicines Agency (EMA) and United States Food and Drug Administration (FDA) have both issued separate Letters of Support (LOS) to encourage the further development and exploratory use of percent change from baseline of the following urinary markers: alpha-glutathione S-transferase (α-GST), clusterin (CLU), cystatin C (CysC), kidney injury molecule-1 (KIM-1), neutrophil gelatinase-associated lipocalin (NGAL), osteopontin (OPN), albumin (ALB) and total protein (TPRO) as biomarkers of drug-induced renal tubular injury in early clinical trials. The EMA also supports the use of serum cystatin C utilizing the percent change from baseline approach proposed by the DIKI group. These Letters of Support are based on data submitted by the Innovative Medicine Initiative’s (IMI) Safer and Faster Evidence-based Translation Consortium (SAFE-T) and the Critical Path Institute’s (C-Path) Predictive Safety Testing Consortium’s (PSTC) Nephrotoxicity Working Group (HWG).
These letters describe FDA’s and EMA’s thoughts on the value of α-GST, CLU, CysC, KIM-1, NGAL, OPN, ALB and TPRO and encourages further evaluation. The FDA’s LOS encourages “the exploratory use of these urinary biomarkers (α-GST, CLU, CysC, KIM-1, NGAL, OPN, ALB, and TPRO) as biomarkers of renal tubule injury in early clinical trials.”
Additionally, the FDA stated that the “exploratory human data suggests that the candidate drug-induced renal tubular injury biomarkers may be more sensitive and specific for the detection of acute kidney injury, especially when used in combination, than traditional means of monitoring for nephrotoxicity. In addition, you observed a rise in most of the candidate biomarkers (urinary a-GST, CLU, KIM-1, NGAL, CysC, and OPN) that preceded a clinically-relevant rise in sCr.”
The Summary Data Package contains summary statistics, description of analysis methods, and results tables submitted to the EMA and U.S. FDA.
In 2018, The US Food and Drug Administration (FDA) issued its first ever qualification of a clinical safety biomarker based on data submitted jointly by the Foundation for the National Institutes of Health (FNIH) Biomarkers Consortium (BC) and the Critical Path Institute’s (C-Path) Predictive Safety Testing Consortium (PSTC).
The qualification applies to a single composite measure (CM) of six urine biomarkers, to be used in conjunction with traditional measures of kidney function; the six safety biomarkers include clusterin (CLU), cystatin-C (CysC), kidney injury molecule-1 (KIM-1), N-acetyl-beta-D-glucosaminidase (NAG), neutrophil gelatinase-associated lipocalin (NGAL), and osteopontin (OPN).
The purpose and Context of Use (COU) for this qualified biomarker are as follows: A safety composite biomarker panel to be used in conjunction with traditional measures to aid in the detection of kidney tubular injury in phase 1 trials in healthy volunteers when there is an a priori concern that a drug may cause renal tubular injury in humans.
Additional details are available in the User’s Guide: Kidney Safety Composite Measure Biomarker for Use in Clinical Development.
There is the opportunity for a paradigm shift in drug development and discovery to implement the use of complex in vitro models (CIVMs) such as micro physiological systems (MPS), spheroids, organoids, or organ/tissue on a chip. The need and desire have been outlined in recent publications (Avila et al, 2023, Tomlinson et al, 2023) and there are initiatives addressing this from different stakeholder groups. Therefore, in 2023 C-Path, with funding from the US FDA, launched a public, collaborative project that includes scientists impacted as drug developers, model developers, or reviewers to (1) develop an evidentiary framework for CIVMs through workshops and publications, (2) draft relevant contexts of use to initiate qualification, and (3) advance implementation of CIVMs in regulatory decision-making.
In the first year of this FDA Broad Agency Announcement (BAA) project, scientists from the pharmaceutical industry, CIVM companies, and health authorities discussed and debated components for a qualification framework to assist in developing appropriate contexts of use to gain regulatory acceptance. This culminated with a public workshop held in September 2023. Sessions included developing contexts of use, analytical considerations for establishing CIVM and system performance, and biological performance considerations for CIVM with appropriate test compounds and biomarker endpoints. You can view the final agenda and slides or watch video recordings of the workshop here.
Workshop participants and additional scientists are drafting a qualification framework whitepaper which will be publicly available in the second half of 2024. Finally, a list of draft contexts of use were summarized for application in assessing suspected drug-induced liver injury using CIVMs.
A second (2024) and third (2025) public, in-person workshop will be held to refine the framework and address aspects specific to additional organs. The second workshop will be held September 26-27, 2024. Day one’s focus is on general aspects applicable across organs and models and day two will delve into breakout groups for organ-specific components. The meeting will include sessions from key opinion leaders in model development, drug development, and clinical safety on general considerations for qualification, as well as interactive breakout sessions focusing on specifics related to different organ systems and disease models. You can register for the 2024 workshop here.
The framework will permit a clear path for future projects by PSTC to advance CIVMs for regulatory acceptance. PSTC plans to initiate qualification of CIVMs based on the recommendations developed from the workshops and framework.
Paul Watkins, M.D.,
University of North Carolina, Eshelman School of Pharmacy, Institute for Drug Safety Sciences
Mitchell McGill, Ph.D.,
University of Arkansas for Medical Sciences
Roscoe Warner, Ph.D.,
University of Michigan Medical School, Department of Pathology
Kent Johnson, M.D.,
University of Michigan Medical School, Department of Pathology
Nicholas King, MS,
Executive Director, Predictive Safety Testing Consortium
Graham Marsh, PhD,
Scientific Director, Predictive Safety Testing Consortium
Katrina Peron, MS,
Senior Project Manager, Predictive Safety Testing Consortium
Laura Riley, MPH,
Data Manager III
Laura Lummus, BFA, PACE,
Senior Project Coordinator, Predictive Safety Testing Consortium