Save the Date for C-Path’s Premier Global Impact Conference
We are thrilled to invite you to the inaugural Critical Path Institute Global Impact Conference taking place this September...


Autosomal Dominant Polycystic Kidney Disease (ADPKD) is a debilitating genetic disease affecting more than 600,000 Americans and 12 million people worldwide. Currently, only one FDA-approved drug (Tolvaptan) exists for ADPKD, but given its mechanism of action and side effects profile (liver toxicity, polyuria), the need for an improved therapy remains a priority.
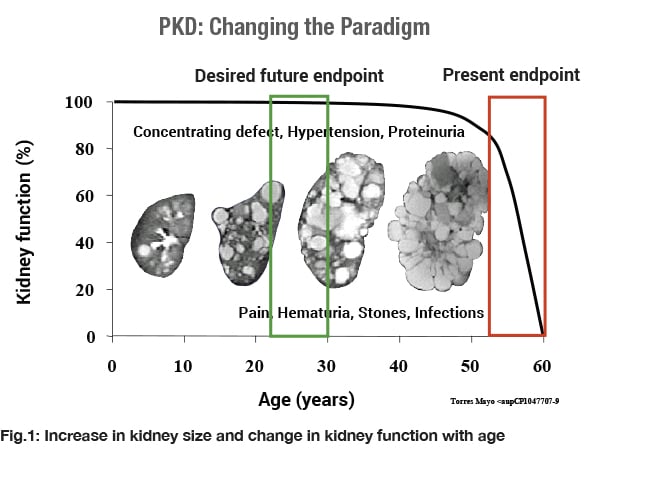
The clinical course of kidney disease in ADPKD is typically marked by a long period of stable glomerular filtration rate (GFR) due to hyperfiltration despite the continuous expansion of height-adjusted total kidney volume (htTKV) due to the growth of cysts. Due to the observed stability or slow decrease of GFR even in the presence of ~5-fold change in kidney volume in many patients, clinical trial design in ADPKD is challenging, especially for earlier disease stages.
There is a critical need for a biomarker that will assess disease progression at an earlier stage, when patients may be more likely to respond to new therapies, and before they have incurred serious, irreversible damage.
The initial goals of C-Path’s PKD Consortium were to develop CDISC (Clinical Data Interchange Standards Consortium) data standards for PKD and to gather clinical data from ADPKD patients collected over many years in patient registries.
Using the data collected, a disease model that evaluated the relationship between baseline Total Kidney Volume (TKV) and progression of ADPKD was developed.
The models, done in conjunction with the PKD Foundation, academic, industry and regulatory stakeholders, were used to support the regulatory qualification of baseline TKV (with or without age inclusion) as an enrichment prognostic biomarker for ADPKD, to predict a 30% decrease in eGFR.
PKDOC has successfully qualified Total Kidney Volume as a prognostic enrichment biomarker with both the Food and Drug Administration (FDA) and the European Medicines Agency (EMA). Furthermore, FDA designated TKV as a reasonably likely surrogate marker for disease progression in ADPKD. This marker could serve as an endpoint under an accelerated approval pathway followed by a post-marketing confirmation trial showing an effect on the loss of kidney function.
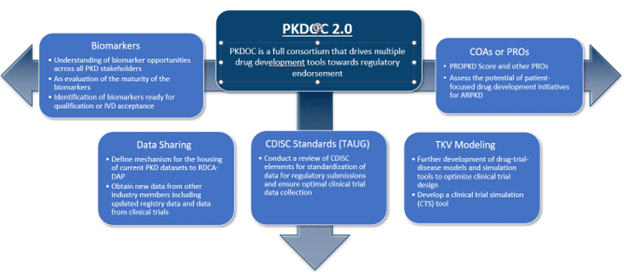
The current projects at PKDOC are focused on development of a clinical trial simulator (CTS) tool for ADPKD, identification and qualification of novel prognostic and drug response biomarkers, and development of COA/PRO tools. Attendees include biopharmaceutical companies, academic organizations, foundations, patient advocacy groups, and regulatory agencies from around the world. Anyone who is interested may participate.
The Polycystic Kidney Disease Outcomes Consortium database consists of de-identified data from three longitudinal observational patient registries. The data have been standardized and aggregated into a common format using a Clinical Data Interchange Standards Consortium (CDISC) Standard Data Tabulation Model (SDTM) structure. This enables analyses to be performed on a larger expanded dataset. The data cover approximately seven decades of patient visits from the following sources:
The common database contains data from a total of 2498 subjects with ages ranging from 0 to 84 years at study entry (mean 35.9; median 37). A majority (81%) of the subjects are Caucasian, and 59% are female. There were 252 deaths and 667 ESRD events recorded with varying levels of timing information (ranging from the precise event day to just the event year). Because the data are from patient registries and not clinical trials, the timing of the visits is irregular, and not all tests or measurements were done on a consistent basis. Nonetheless, this is a rich dataset of historical data of patients with Autosomal Dominant PKD.
Welcome to the information page for the PKD Joint Modeling clinical trial simulation tool developed by the Polycystic Kidney Disease Outcomes Consortium (PKDOC).
PKDOC would like to acknowledge the leadership and contributions of the following team members:
This drug development tool was developed by linking longitudinal TKV measurements to the probability of a 30% decline of estimated glomerular filtration rate (eGFR) or end-stage renal disease. This tool is intended for use in drug development through use of TKV as a prognostic biomarker to select patients for clinical trials of new therapies for ADPKD. For additional details, please see the following publication: “A Drug Development Tool for Trial Enrichment in Patients With Autosomal Dominant Polycystic Kidney Disease” .
To access the tool files, or if you have any questions relating to the tool, please contact Wendy Vanasco at wvanasco@c-path.org.
On August 31, 2015, C-Path announced that the U.S. Food and Drug Administration (FDA) had issued a qualification decision in the form of a draft guidance to C-Path’s Polycystic Kidney Disease Outcomes Consortium (PKDOC) for total kidney volume (TKV) as a prognostic biomarker to select patients for clinical trials of new therapies for Autosomal Dominant Polycystic Kidney Disease (ADPKD). For further information on this decision, follow the links below:
On November 13, 2015, C-Path announced that the European Medicines Agency (EMA) rendered a positive qualification opinion to C-Path’s Polycystic Kidney Disease Outcomes Consortium for total kidney volume (TKV) as a prognostic biomarker to select patients for clinical trials of new therapies for Autosomal Dominant Polycystic Kidney Disease (ADPKD). For more information, see the document linked below:
Total Kidney Volume is a measurement of the size of the kidneys and has been shown to be predictive of a future decline in kidney function. This draft guidance provides qualification recommendations for the use of TKV, measured at baseline, as a prognostic enrichment biomarker to select patients with ADPKD at high risk for a progressive decline in renal function (defined as a confirmed 30% decline in the patient’s estimated glomerular filtration rate [eGFR]). The use of TKV as a biomarker—along with the patient’s age and baseline eGFR—can help those conducting clinical trials in finding appropriate candidates, potentially improving the accuracy and efficiency of those trials.
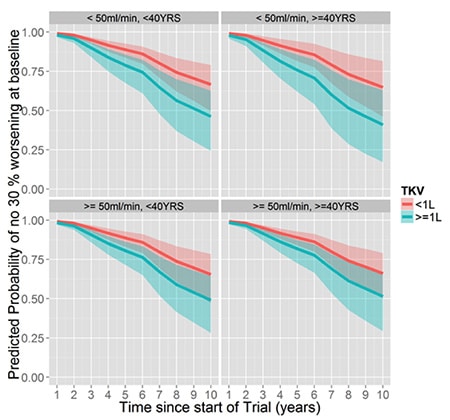
This set of graphs show clearly that patients with large kidney (greater than one liter in volume) have a greater likelihood of progressive kidney failure over time, regardless of age (younger or older than 40 years old) or baseline estimated Glomerular Filtration Rate (more or less than 50 ml per minute).
Age is predictive of kidney function decline when kidneys are larger and baseline kidney function is low:
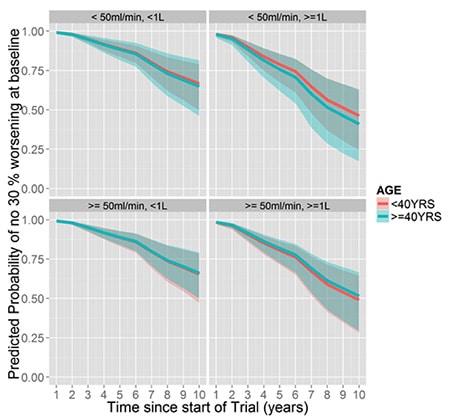
Baseline kidney function is predictive of kidney function decline when kidneys are large and the patients are older:
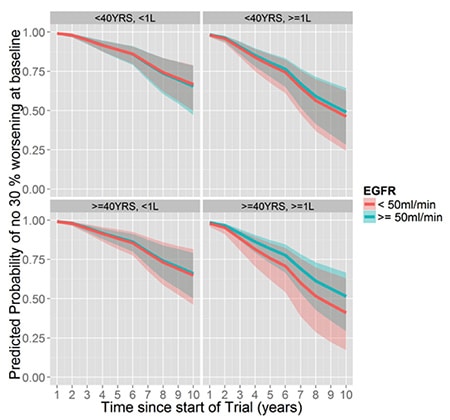
Baseline Total kidney volume is predictive of kidney function decline regardless of age or baseline kidney function:
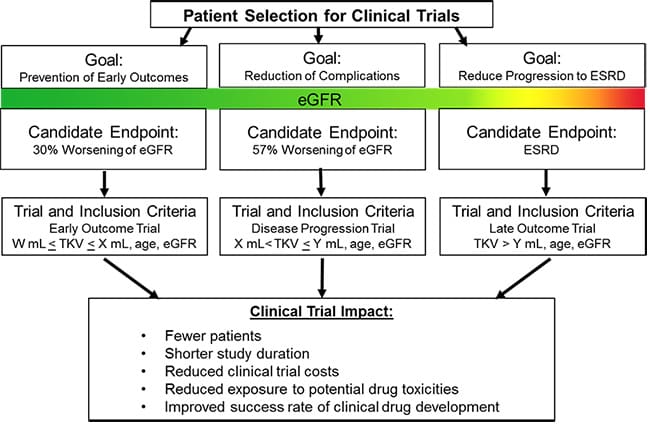
This is a simplified flow chart that shows how TKV can be used for patient selection in various stages of drug development and the anticipated benefits.
Sorin Fedeles, PhD, MBA
Executive Director, Polycystic Kidney Disease Outcomes Consortium
Matt Becka, MBA, DNP, RN
Chief Research Officer, PKD Foundation
Frank Czerwiec, MD PhD
Chief Medical Officer, Sparrow Pharmaceuticals
Ronald Perrone, MD
Scientific Director, Clinical and Translational Research Center, Professor, Tufts University
Sorin Fedeles, PhD, MBA
Executive Director, Polycystic Kidney Disease Outcomes Consortium
Wendy Vanasco
Rare and Orphan Diseases, Polycystic Kidney Disease Outcomes Consortium
Robert Stafford, MA
Senior Data Manager, Data Management Team Lead, Data Collaboration Center