Critical Path Institute Announces New Board Members Bonnie A. Allin and Louis Breton
Critical Path Institute (C-Path) today announced the appointments of Bonnie A. Allin and Louis Breton to its Board of Directors.


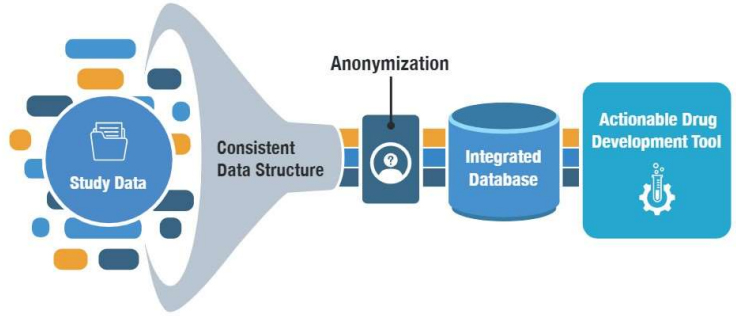
The Data Collaboration Center provides the resources, services and solutions to enable multiple organizations to collaborate in a neutral setting to share data, which in turn creates new solutions and tools that accelerate drug development.
Data is crucial for understanding the mechanisms of disease and identifying effective treatments. Without sufficient data, medical researchers are unable to fully explore the complexities of disease, and this can severely limit our ability to make progress in the field.
Lack of data hurts medical progress by making it more difficult to identify patterns and correlations between different factors that contribute to disease. For example, if researchers only have access to data from a limited number of patients or other sources, they may not be able to identify important risk factors or environmental exposures that contribute to the progression of a particular disease. This can lead to missed opportunities for prevention or treatment.
The Data Collaboration Center (DCC) was founded by C-Path to generate secure, large-scale data solutions for medical research, and provide unsurpassed expertise in curating, standardizing, analyzing and sharing medical data from around the world. We operate in a neutral space with a focus on accelerating clinical research and improved treatments by maximizing the utility of medical data. This is accomplished through robust data management and curation processes, and through the development and application of data standards and ontologies, as well as custom-built tools. The DCC possesses the top-tier technical expertise and project management resources to support advanced research efforts.
Through the collaborative environment created by this program, the DCC provides the following:
The CAMD AD/MCI (Critical Path for Alzheimer’s Disease Consortium Database) is a unified clinical trial database for Alzheimer’s disease. CPAD has a mission to develop new technologies and methods to accelerate the development and review of medical products for neurodegenerative diseases.
CPAD is focused on accelerating therapeutic treatment development for patients with chronic neurodegenerative disease, namely, Alzheimer’s disease (AD), the most prevalent and devastating dementia, by advancing drug development tools (DDTs) and when appropriate, medical device development tools (MDDTs) for evaluating drug efficacy, conducting clinical trials, and streamlining the process of regulatory review. The consortium focuses on sharing precompetitive patient-level data from the control arms of legacy clinical trials, developing new tools to be submitted to the regulatory agencies, and developing consensus data standards.
CAMD has the following areas of focus: (1) qualification of objective biomarkers, including both biochemical and observational digital biosensor measures of health, (2) development of common data standards, (3) creation of integrated databases for clinical trials data, and (4) development of quantitative model-based tools for therapeutics development.
In February 2019, the Friedreich’s Ataxia Research Alliance (FARA) and Critical Path Institute (C-Path) formed a collaborative partnership to create the Friedreich’s Ataxia Integrated Clinical Database (FA-ICD). This database was designed to catalyze and accelerate Friedreich’s ataxia research and drug development by curating and standardizing FA clinical trial and natural history data, and making these data available to qualified researchers. These researchers can access and analyze data in aggregate, or filter and view individual de-identified patient-level data from several clinical trials and a large FA natural history study.
Today the FA-ICD continues to grow and has been integrated into C-Path’s new Rare Disease Cures Accelerator Data and Analytics Platform (RDCA-DAP) which is focused on rare disease research. RDCA-DAP hosts data from many rare diseases and fosters research across diagnoses, removing silos that can limit progress. As more data is being shared with RDCA-DAP, Data Managers within the DCC curate and standardize these data to a common data model, allowing for a smoother transition to research and analysis. For more information about RDCA-DAP or the FA-ICD data, please visit our RDCA-DAP page.

The Integrated Parkinson’s Database includes integrated and standardized data from both PD observational studies and randomized clinical trials and contains thousands of participants’ data, anonymized and deidentified. The database covers over 600 variables, such as demographic information, clinical measures, medication usage data, dropouts, and more.
The CPP integrated database is rich in patient-level outcome measures and includes item level patient level data. It contains limited biomarker and genetics data and does not include data collected using digital health technologies. In accordance with the Data Contribution Agreements between CPP and data contributors, CPP is obligated to protect the identity of the individual datasets and provides access to the datasets in aggregate with masked study identifiers.
We would like to thank our collaborators for their generous contributions to the CPP integrated Parkinson’s Database. View acknowledgements.
The Multiple Sclerosis Outcome Assessments Consortium (MSOAC) Placebo Database presently includes 2465 individual patient records from 9 clinical trials. This version 1.0 includes records from relapsing-remitting, secondary progressive, and primary progressive forms of MS. Placebo arms from clinical trial datasets, which were contributed by industry members of MSOAC, are aggregated in the MSOAC Placebo Database. The MSOAC Placebo Database presently includes 2465 individual patient records from 9 clinical trials. This version 1.0 includes records from relapsing-remitting, secondary progressive, and primary progressive forms of MS.
PKD (Polycystic Kidney Disease Consortium Database) is a standardized database of aggregated data from three longitudinal observational patient registries.
The Polycystic Kidney Disease (PKD) Outcomes Consortium is a successful collaboration between Critical Path Institute (C-Path), the PKD Foundation, Clinical Data Interchange Standards Consortium (CDISC), four leading academic medical centers (Tufts University, University of Colorado Denver, Emory University, and Mayo Clinic), and three pharmaceutical companies. Its mission is to develop tools and promote research that will lead to the discovery of treatments for PKD and improve the lives of all it affects. The Consortium is led by C-Path and funded through a grant from the PKD Foundation and philanthropic donations. Additionally, a representative from the U.S. Food and Drug Administration (FDA) serves as an active advisor to the Consortium.
The Rare Disease Cures Accelerator-Data and Analytics Platform (RDCA-DAP®) promotes the sharing of existing patient-level data and encourages the standardization of new data collection. By integrating data in a format suitable for analytics, RDCA-DAP accelerates the understanding of disease progression (including sources of variability to optimize the characterization of subpopulations), clinical outcome measures and biomarkers, and facilitates the development of mathematical models of disease and innovative clinical trial designs.
The TB-APEX data platform is designed to catalyze and accelerate TB research by curating and standardizing preclinical study data and making this data publicly available to qualified researchers.
The TB-PACTS data platform is designed to catalyze and accelerate tuberculosis (TB) research by making aggregated Phase III TB clinical trial data publicly available to qualified researchers. TB-PACTS represents a collaborative partnership between the Special Programme for Research and Training in Tropical Diseases (TDR), the TB Alliance, St. George’s University of London, Case Western University, the British Medical Research Council and Critical Path Institute (C-Path).
To enable multiple organizations to work together in a neutral setting and share medical data in order to optimize its value in creating new insights and tools that accelerate drug development in areas with unmet healthcare needs.

Since 2005, C-Path has been a trusted and independent entity specializing in biomedical research collaboration and regulatory science. We have become a go-to provider of data sharing and analysis solutions for stakeholders from industry, global regulatory agencies, government agencies, non-governmental organizations, patient advocacy groups, and academia. C-Path has over a decade of experience in data science, data standards, data management, data security, and patient privacy data platform development. Today, C-Path’s DCC-managed data platforms securely host data from over 165 clinical trials, over 160 nonclinical studies, as well as genomic data, imaging data, real-world data, and other data types, representing over 150,000 data subjects, over 215 million data points, and a multitude of different therapeutic areas, diseases, and conditions.
The DCC is grateful to the many partner organizations that have contributed to our success over the years and who share and support the vision of accelerating the drug development process through collaboration and data sharing. We’re proud to showcase the partners who have contributed to the advancement of our mission.
The DCC has a comprehensive data privacy program which encompasses all jurisdictions in which we manage subject data. This program includes policies around individual patient-level data that meet or exceed human subject research protection requirements as well as applicable regulatory policy. Every contribution of clinical data to a DCC project is governed by a Data Contribution Agreement (DCA), which specifies the scope of data sharing permitted by the contributor. Data contributors must also certify that they have met all applicable requirements to enable secondary research on contributed data. Through the support of our sponsors, members, and collaborators, data permitted to be shared through the DCA are currently made freely available to qualified researchers. All data are encrypted in rest and in transit, accessed only for qualified research purposes governed by data use agreements.
In addition to internal controls ensuring data is secure and patient privacy is protected, C-Path’s new data and analytics platform, the CP-DAP, is built in the Digital Research Environment (DRE) of Aridhia Informatics. The DRE maintains ISO 27001 certification and is HITRUST certified. Additional certifications and security details about the DRE utilized by C-Path can be found on their Security and Compliance page.
Rick Liwski
Chief Technical Officer, Director of DCC
Vicki Theurer Crider
Senior Project Manager
Kashawna Orme
Senior Project Coordinator
Corissa Lau
Project Manager II
Ramona Walls, PhD
Executive Director of Data Science
Nicole Vasilevsky, PhD
Associate Director of Data Science
Daniel Olson, MPH
Standards and Ontology Engineer
Emily Hartley
Data Engineer
Ian Braun, PhD
Data Scientist
Kurt Michels, PhD
Data Analyst
Mike Pauley
Data Engineer
Pavan Kumar Sudhakar
Data Engineer
Roopal Bhatnagar, MS
Data Analyst II
William Roddy
Data Engineer Team Lead
Laura Song
Associate Director, DCC
Diane Corey
Data Team Manager; Standards Developer
Albert Barrera
Data Manager
Robert Stafford, MA
Data Management Team Lead
Adam Regalski
Data Manager
Alejandro Lozano
Data Manager II
Dan Hartley, MS
Data Manager IV – Senior
Eric Frey
Data Manager IV – Senior
Frank Walker
Data Manager III
German Soto
Data Manager II
Laura Riley, MPH
Data Manager III
Mussie K. Akalu, MSc
Data Manager III
Nathan Cunicelli
Data Manager
Stephanie Marsh
Data Manager II
Patrick O’Meara
Data Team Manager, Work Package Lead
Ahmad Faizan
Data Manager III
Alysha Taylor
Data Engineer
Fionnuala Murphy
Data Manager II
Keith Scollick
Cloud Platform Engineer