C-Path to Spearhead New Task Force Dedicated to Accelerating Drug Development for Progressive Supranuclear Palsy
Critical Path Institute (C-Path) today announced the formation of a new task force...


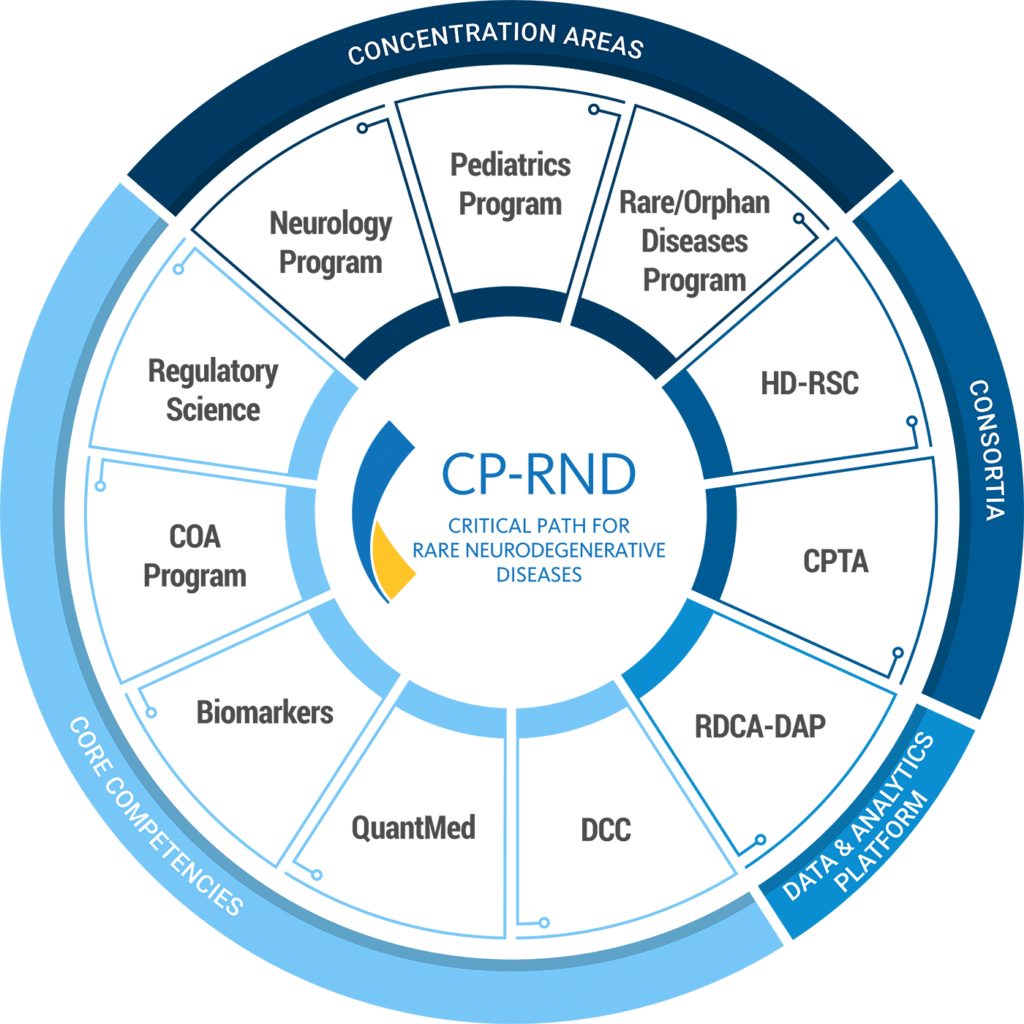
CP-RND was founded in September 2022 to lead collaborations that advance treatments to improve the lives of those affected by rare neurodegenerative diseases, including ALS.
Neurodegenerative diseases occur when nerve cells in the brain or peripheral nervous system progressively lose structure and/or function and ultimately die. Significant challenges remain in research and drug development for rare neurodegenerative diseases and advances are needed to improve the lives of those affected.
Critical Path for Rare Neurodegenerative Diseases (CP-RND), brings together multiple experts in rare neurodegenerative diseases, including ALS, as well as biopharmaceutical companies, regulators, patient communities and advocacy organizations to accelerate and advance our understanding of disease pathology, treatment options, diagnostics and drug development.
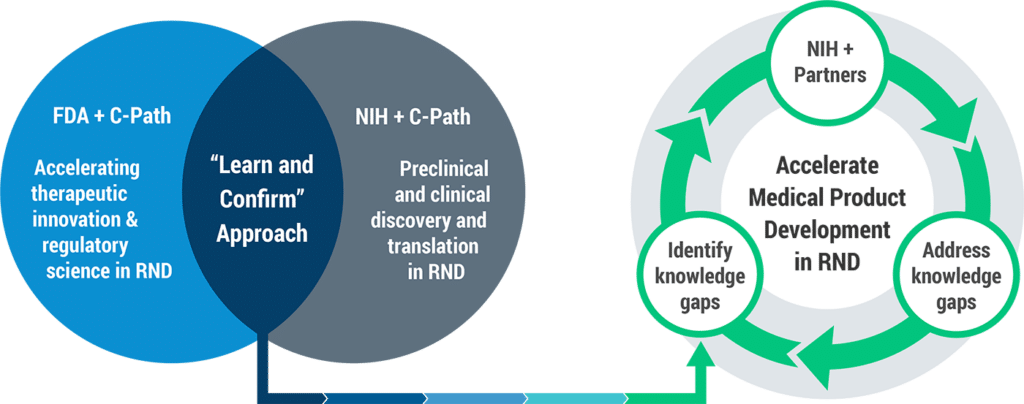
C-Path has built a robust infrastructure within a pre-competitive framework that provides a safe harbor for commercial drug developers to bring data and shared learnings. This, combined with independent efforts from the FDA and NIH, provides a prime opportunity and unique global venue for accelerating drug and therapeutic development in rare neurodegenerative diseases. CP-RND also facilitates interactions and collaboration with the FDA and NIH, as well as the external biomedical research community to discuss cross-cutting clinical and regulatory policy issues, and foster consistent decision making.
CP-RND is focused on increasing our understanding of disease pathogenesis and natural history by quantifying disease progression. These efforts include evaluating potential biomarkers from patient-level data and optimizing clinical trial design, including exploration of innovative trial designs, to improve the efficiency and success of drug development.
This, together with the capabilities of C-Path’s Rare Disease Cures Accelerator-Data and Analytics Platform (RDCA-DAP) integrates multiple patient-level data sources across rare diseases, providing the foundation for leveraging advances in basic and clinical sciences.
CP-RND aims to enhance the medical advances for rare neurodegenerative diseases, including ALS, by:
Interested in participating in this collaboration? Please inquire and include your contact information at CP-RND@c-path.org.
ALS is a relentless disorder characterized by the degeneration of both upper and lower motor neurons, leading to progressive weakness and paralysis. In approximately 30% of cases, non-motor neurons in the frontal and temporal regions of the brain are also affected, causing impairments in various cognitive domains. The disease is uniformly fatal, with an average survival of only about three years after diagnosis, although some individuals succumb even faster and others survive for more than a decade. About 10% of ALS cases are inherited—typically as a dominant trait linked to a growing number of ALS-causing genes (“familial” ALS), while the remaining cases have no apparent family history of ALS (“sporadic” ALS). The cumulative lifetime risk for ALS is estimated at 1:400, which predicts that more than 800,000 individuals now alive in the US will develop ALS.
CP-RND actively engages the ALS community through conference and participation, hosting Discussion Forums regularly throughout the year, and participation in collaborative efforts convened by other ALS community stakeholders. CP-RND will focus its efforts on landscaping activities to inform the design of new research projects supported through this initiative, generation of standards, and creation of drug development tools (biomarkers, digital health technologies, trial simulation tools, biological disease classification, novel COAs aimed at accelerating clinical trials in all potential persons impacted with ALS.
Klaus Romero, MD, MS
Chief Executive Officer, Chief Science Officer
Collin Hovinga, PharmD, MS,
FCCP, Vice President, Rare and Orphan Disease Programs
Rick Liwski
Chief Technology Officer; Director, Data Collaboration Center
Terina Martinez, PhD
Executive Director, Critical Path for Rare Neurodegenerative Diseases and Critical Path to Therapeutics for the Ataxias
Thomas Hart, JD
Director of Outreach, Critical Path for Rare Neurodegenerative Diseases
Katie O’Keefe, MS
Associate Director, Critical Path for Rare Neurodegenerative Diseases
Tanya Williams, MPH
Sr. Project Manager, Critical Path for Rare Neurodegenerative Diseases
Ramona Walls, PhD
Executive Director, Data Science
Laura Hopkins, MS, MLS
Associate Director, Rare and Orphan Diseases Program
Stephanie Irvin
Project Coordinator