Access the FA-ICD
Friedreich’s ataxia (FA) is a debilitating, life-shortening, degenerative neuromuscular disorder. It is the most common form of hereditary ataxia, affecting approximately 1 in every 50,000 people in the United States and Europe (FA is primarily found in white, Hispanic, and Southeast Asian populations.
Friedreich’s Ataxia Integrated Clinical Database (FA-ICD)
Friedreich’s ataxia (FA) is a debilitating, life-shortening, degenerative neuromuscular disorder. It is the most common form of hereditary ataxia, affecting approximately 1 in every 50,000 people in the United States and Europe (FA is primarily found in white, Hispanic, and Southeast Asian populations; incidence is very rare in other racial groups). FA is an autosomal recessive, single gene disorder, caused by mutations in the FXN gene. Loss of balance and coordination is the most common presenting symptom typically beginning between the ages of 5 and 15 years with progression of symptoms leading to loss of ambulation and independence of all activities of daily living. Adult or late onset FA is less common, affecting <25% of diagnosed individuals, and can occur anytime during adulthood. While neurological features of the disease are fully penetrant, affecting 100% of those diagnosed, it is a multi-system disease. Two thirds of patients also develop cardiomyopathy, more than half develop severe scoliosis, and 10-20% develop diabetes. The mean age at death is 35 years due to cardiac complications in greater than 50% of individuals.
More information can be found at https://curefa.org
FA-ICD Mission:
Launched in February 2018, the Friedreich’s Ataxia Integrated Clinical Database (FA-ICD) is designed to catalyze and accelerate Friedreich’s ataxia (FA) research and drug development by curating and standardizing FA clinical trial and natural history data into CDISC format and making this data publicly available to qualified researchers. These researchers can access and analyze data in aggregate, or filter and view individual de-identified patient-level data from four clinical trials and a large FA natural history study. Additional data may be available in the future.
FA-ICD Partnership:
This initiative represents a collaborative partnership between the Friedreich’s Ataxia Research Alliance (FARA) and the Rare Diseases Cures Accelerator Data and Analytic platform (RDCA-DAP) of the Critical Path Institute (C-Path), with a goal of expanding the FA-ICD platform by engaging with other data contributors to secure additional datasets.
FA-ICD Content:
Approved researchers can access de-identified patient-level data from placebo arms of four clinical trials and a natural history study, including:
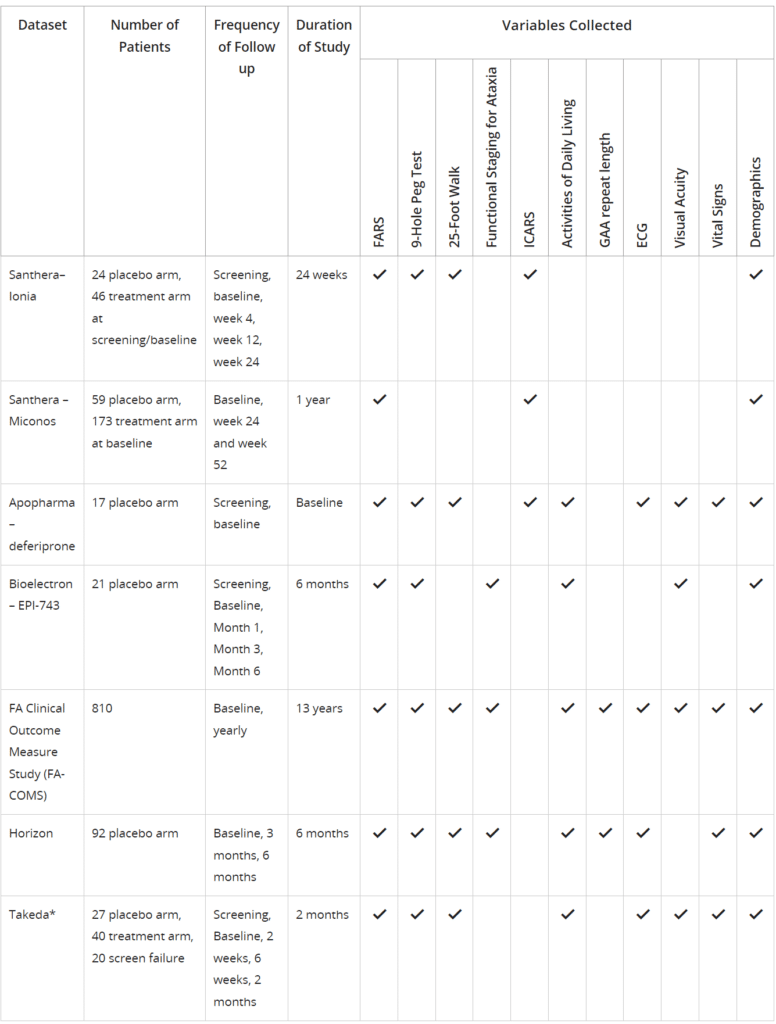
*Data cannot be shared with external users
Trial data is anonymized in the database, so researchers will not be able to ascertain which data came from which trial, and they will not be able to identify participants in any study.
FA-ICD also contains data from the FARA-funded Friedreich’s Ataxia Clinical Outcome Measure Study conducted by the Collaborative Clinical Research Network (CCRN). The CCRN has collected natural history data on over 1,000 FA patients. The natural history for over 500 patients goes back more than 5 years and has more than 250 patients with baseline visits before 18 years of age.
Information about these studies may be found by clicking here.
This dataset is updated on an annual basis as the study is still ongoing with new data being prospectively accrued.
FA-ICD Access and Data Contribution:
FA-ICD catalogs completed FA clinical trials and natural history data and makes it available to qualified researchers. Access to the patient level data is by request only and subject to review and approval by the FA-ICD Steering Committee. To request access, you must agree to the Terms and Conditions for Use and submit a Request for Access application detailing how the data will be used, who will access the data and any plans for publishing work informed by the data. The Terms and Conditions can be accessed here.
FA-ICD encourages data contributions from interventional and non-interventional studies and is always willing to discuss how companies or other researchers can engage with the initiative. For more information on FA-ICD, including “Frequently Asked Questions” and how your organization can contribute data, please contact Alexandre Betourne, abetourne@c-path.org or rdcadap@c-path.org.
FA-ICD Solutions:
The FA-ICD is currently being utilized to develop nonlinear mixed effects-based models of disease progression in FA to investigate and compare available outcome measures collected in interventional and non-interventional studies. Additionally, a placebo effect model may be incorporated to quantify the magnitude, onset, and offset of the placebo response for control arm subjects included in the FA-ICD. These models are intended to provide the foundation for a downstream clinical trial simulation tool.
Access the FA-ICD platform
The platform for FA-ICD is hosted by the Critical Path Institute Online Data Repository (CODR) and by the Rare Disease Cures Accelerator – Data and Analytics Platform.
For access to the FA-ICD through RDCA-DAP, please visit https://c-path.org/programs/rdca-dap/ for more information and for any questions, please email rdcadap@c-path.org.
C-Path has a decade of experience in data standards development, platform development and hosting, patient-level data privacy stewardship, data platform security, and controlled access methodology.
C-Path currently provides secure hosting for data collected from more than 100 clinical trials, over 60,000 subjects, and nine different therapeutic areas, totaling more than 200 million data points.
Important information about FA-ICD content and access:
- The data platform contains, but is not limited to:
- Demographic data
- Friedreich’s Ataxia Rating Scale (FARS)
- International Co-operative Ataxia Rating Scale (ICARS)
- Activities of Daily Living Scale
- Functional Disability Scale
- 25-Foot Walk
- 9-Hole Peg Test
- Modified Fatigue Impact Scale (MFIS)
- MOS Pain Effects Scale (PES)
- Bladder Control Scale (BLCS)
- Bowel Control Scale (BWCS)
- Impact of Visual Impairment Scale (IVIS)
- Sloane low contrast letter acuity scale. (LCLA)
- SF-10 and SF-36
- Vital signs
- ECG
- Echocardiogram
- Genetic mutation
- C-Path has fully anonymized all data.
- Researchers must agree to the Terms and Conditions for Use of the FA-ICD data platform and submit an online application form to request access to the data platform.
- The FA-ICD Steering Committee approves data access for external users.
- The Resources tab within FA-ICD contains information to help users understand and make use of the platform capabilities.
Important information about data standardization:
- C-Path has normalized all data to the CDISC Study Data Tabulation Model (CDISC SDTM) to enable researchers to analyze the data in aggregate.
- FA-ICD provides basic information on how data are structured using CDISC. Knowledge of SDTM is required for effective use of the data. Information and training about SDTM are available through the CDISC website; researchers who receive access to FA-ICD will find a link to the CDISC website on the Resources tab.
A summary of detailed concepts captured by SDTM domains contained in the FA-ICD is provided in the table below.
| CDISC Domain | Contents |
| CE | Clinical events |
| CM | Medications |
| CV | LVEF, LVSF, LVMass, LVIDD, LVIDS, IVS, ejection fraction, fractional shortening, valve regurgitation, wall motion, wall thickness, LVOT, LVIT, interpretation |
| DD | Age at death, autopsy indicator, death certificate obtained, hospital medical record obtained, cause of death |
| DM | Age, gender, race, ethnicity, trial arm, country |
| DS | Withdrawal, death, lost to follow up, reconsent |
| DU | Assistive walk device indicator, type, age |
| EG | Mean heart rate, PR, QRS duration, QT, QTc, interpretation |
| FA | Occurrence and completion indicators, reason for missing visit |
| FT | FARS*, 25-Foot Walk, 9-Hole Peg Test, Functional Staging for Ataxia |
| LB | ALT, AST, creatinine, corrected leukocytes, ferritin, glucose, hemoglobin, neutrophils, neutrophils/leukocytes, platelets, nucleated erythrocytes/leukocytes, leukocytes, zinc |
| MH | Medical history events |
| OE | Letter eye chart, cataract surgery laterality, require correction for vision indicator |
| PE | Physical exam |
| PR | Scoliosis surgery, cardiac procedures |
| QS | Activities of Daily Living, SF-10, SF-36, PGI, BLCS, BWCS, MFIS, IVIS, PedsQL, PES |
| RE | FEV1, FEV1/FVC, FVC, FEF25-75, percent predicted, indication, interpretation |
| RP | Pregnancy confirmed, birth control method, pregnancy outcomes |
| RS | International Co-operative Ataxia Rating Scale (ICARS)* |
| SC | Level of education, living status, marital status, occupation |
| SS | Change in ambulation status |
| VS | Height, weight, BMI, pulse rate, DBP, SBP, heart rate |
*Note: The FARS is located in the FT domain because the answers to the questions are governed by the duration/number of times a patient could perform the task. The ICARS is located in the RS domain because the answers are more subjectively answered by the clinician/technician who is observing the patient performing the task.

