C-Path Launches Clinical Trial Simulator for Duchenne Muscular Dystrophy Research
Critical Path Institute’s (C-Path) Duchenne Regulatory Science Consortium (D-RSC) is excited to announce the launch of...


Founded in 2015, D-RSC supports collaborative research through shared data access and advances development of drug development tools.
Duchenne Muscular Dystrophy (DMD) is a fatal disease caused by a genetic mutation in the DMD gene and manifests as progressive muscle degeneration leading to loss of ambulation, loss of upper limb function, respiratory and cardiac function failure. A major hurdle when it comes to improving treatments for DMD is the complexity of the disease itself, including the wide variety of genetic mutations. This complexity means that developing effective treatments can be difficult, as researchers must consider multiple factors, such as the specific type of mutation, disease stage and age of individuals living with DMD.
Variable disease progression, and lack of standardized indicators of disease for DMD are also major challenges which can make it difficult to assess treatment effectiveness.
In addition, the rarity of the disease poses a challenge for clinical trials. DMD has approximately 20,000 new diagnoses per year, mostly in boys. The growing number of drugs in development, and the small population size, makes it difficult to recruit enough patients for clinical trials, which can lead to long trial durations and limited statistical power. In turn, this makes it difficult to detect treatment effects.
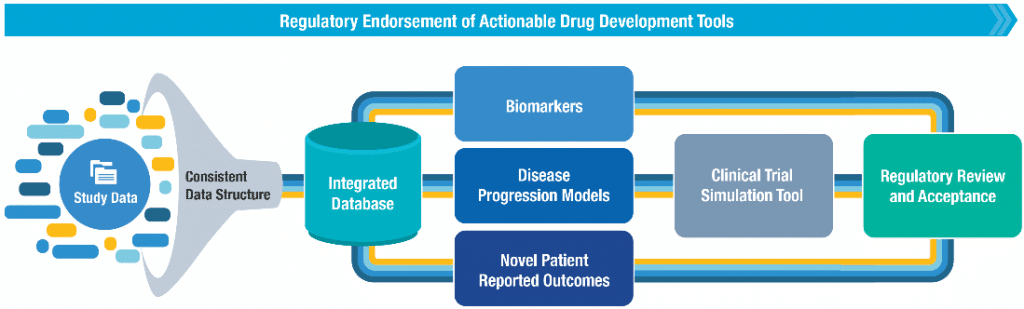
To combat these challenges, the Duchenne Regulatory Science Consortium (D-RSC) has created an integrated database of patient-level clinical data from DMD studies, which is partially available for analysis by the Duchenne community as permitted by the owners of each dataset. Created by Critical Path Institute (C-Path) and Parent Project Muscular Dystrophy (PPMD), D-RSC has generated standard terminology to integrate data and has written the CDISC Duchenne Muscular Dystrophy Therapeutic Area User Guide, which is available to the community. Using its reach database, D-RSC has completed the development of the first Clinical Trial Simulation tool for DMD and recently received a Letter of Support from the EMA while additional regulatory review processes are ongoing. You can access the DMD Clinical Trial Simulation Tool here.
In collaboration with C-Path’s Predictive Safety Testing Consortium, we are developing glutamate dehydrogenase (GLDH) as a safety biomarker for liver toxicity in patients with underlying muscle damage and have received a formal “letter of support” from EMA and positive response to the Qualification Plan for GLDH from the FDA. Future projects may include supporting the regulatory acceptance of other DMD-relevant biomarkers and patient reported outcomes or development of additional models to be leveraged for drug development.
The D-RSC team works every day to mitigate the effects of DMD and to bring new treatments to market faster. To do so, D-RSC provides:
The Duchenne Regulatory Science Consortium (D-RSC) Database is open to non-consortium members if approved by a data use committee consisting of neutral members of the consortium. By sharing this resource, D-RSC aims to extend and amplify the availability of data to accelerate drug development for Duchenne muscular dystrophy. The database includes data from DMD clinical trials, natural history studies, and clinical data collections.
Share Data Here: https://portal.rdca.c-path.org/contribute-data
D-RSC promotes the sharing of patient-level data and encourages the standardization of new data collection. All shared rare disease data, including DMD and BMD, are collected in the Rare Disease Cures Accelerator-Data and Analytics Platform (RDCA-DAP®). By integrating data in a format suitable for analytics, RDCA-DAP accelerates the understanding of disease progression (including sources of variability to optimize the characterization of subpopulations), clinical outcome measures and biomarkers, and facilitates the development of mathematical models of disease and innovative clinical trial designs.
C-Path RDCA-DAP Portal: https://portal.rdca.c-path.org
D-RSC efforts and solutions are all data driven and the precious data shared become part of sophisticated regulatory ready solutions that are provided back to the community in form of freely available drug development tools to advance drug development in DMD and other dystrophinopathies.
What is the DMD Clinical Trial Simulation Tool?
Ramona Belfiore-Oshan, PhD
Executive Director, Duchenne Regulatory Science Consortium
Pat Furlong
Founding President and CEO, Parent Project Muscular Dystrophy
Stacy Owen
Project Manager II, D-RSC
Lysandra Gomez
Project Coordinator II, D-RSC
Klaus Romero, MD, MS, FCP
Chief Executive Officer, Chief Science Officer
Collin Hovinga, PharmD, MS, FCCP, Vice President, Rare and Orphan Disease Programs
Rick Liwski
Chief Technology Officer; Director, Data Collaboration Center
Cécile Ollivier
Vice President, Global Affairs
Shu Chin Ma, PhD, Ma, MS, MPhil,
EMBA, Vice President, Model-Informed Drug Development and Quantitative Medicine
Zihan Cui
Quantitative Medicine Developer II
Grace V. Lee
Post-Doctoral Fellowship, Quantitative Medicine
Sakshi Sardar
Senior Scientific Director, Digital and Precision Medicine, Quantitative Medicine
Lauren Quinlan
Quantitative Medicine Developer I
Rachel Xu
Quantitative Medicine Developer
Yi Zhang
Director of Pharmacometrics, Quantitative Medicine
Diane Corey
Data Team Manager, Data Collaboration Center
Nathan Cuncelli
Data Manager II, Data Collaboration Center